Molecular remedy of complex I defects: rotenone-insensitive internal NADH-quinone oxidoreductase of Saccharomyces cerevisiae mitochondria restores the NADH oxidase activity of complex I-deficient mammalian cells
- PMID: 9689052
- PMCID: PMC21310
- DOI: 10.1073/pnas.95.16.9167
Molecular remedy of complex I defects: rotenone-insensitive internal NADH-quinone oxidoreductase of Saccharomyces cerevisiae mitochondria restores the NADH oxidase activity of complex I-deficient mammalian cells
Abstract
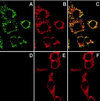
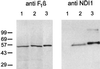
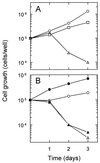
The NDI1 gene encoding rotenone-insensitive internal NADH-quinone oxidoreductase of Saccharomyces cerevisiae mitochondria was cotransfected into the complex I-deficient Chinese hamster CCL16-B2 cells. Stable NDI1-transfected cells were obtained by screening with antibiotic G418. The NDI1 gene was shown to be expressed in the transfected cells. The expressed Ndi1 enzyme was recognized to be localized to mitochondria by immunoblotting and confocal immunofluorescence microscopic analyses. Using digitonin-permeabilized cells, it was shown that the transfected cells, but not nontransfected control cells, exhibited the electron transfer activities with glutamate/malate as the respiratory substrate. The activities were inhibited by flavone, antimycin A, and KCN but not by rotenone. Added NADH did not serve as the substrate, suggesting that the expressed Ndi1 enzyme was located on the matrix side of the inner mitochondrial membranes. Furthermore, although nontransfected cells could not survive in a medium low in glucose (0.6 mM), which is a substrate of glycolysis, the NDI1-transfected cells were able to grow in the absence of added glucose. When glycolysis is slow, either at low glucose concentrations or in the presence of galactose, respiration is required for cells to survive. The mutant cells do not survive at low glucose or in galactose, but they can be rescued by Ndi1. These results indicated that the S. cerevisiae Ndi1 was expressed functionally in CCL16-B2 cells and catalyzed electron transfer from NADH in the matrix to ubiquinone-10 in the inner mitochondrial membranes. It is concluded that the NDI1 gene provides a potentially useful tool for gene therapy of mitochondrial diseases caused by complex I deficiency.
Figures

References
-
- Chomyn A, Mariottini P, Cleeter M W J, Ragan C I, Matsuno-Yagi A, Hatefi Y, Doolittle R F, Attardi G. Nature (London) 1985;314:591–597. - PubMed
-
- Chomyn A, Cleeter M W J, Ragan C I, Riley M, Doolittle R F, Attardi G. Science. 1986;234:614–618. - PubMed
-
- Hatefi Y. Annu Rev Biochem. 1985;54:1015–1069. - PubMed
-
- Yano T, Chu S S, Sled’ V D, Ohnishi T, Yagi T. J Biol Chem. 1997;272:4201–4211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

