Autoregulation of the Raf-1 serine/threonine kinase
- PMID: 9689060
- PMCID: PMC21318
- DOI: 10.1073/pnas.95.16.9214
Autoregulation of the Raf-1 serine/threonine kinase
Abstract
The Raf-1 serine/threonine kinase is a key protein involved in the transmission of many growth and developmental signals. In this report, we show that autoinhibition mediated by the noncatalytic, N-terminal regulatory region of Raf-1 is an important mechanism regulating Raf-1 function. The inhibition of the regulatory region occurs, at least in part, through binding interactions involving the cysteine-rich domain. Events that disrupt this autoinhibition, such as mutation of the cysteine-rich domain or a mutation mimicking an activating phosphorylation event (Y340D), alleviate the repression of the regulatory region and increase Raf-1 activity. Based on the striking similarites between the autoregulation of the serine/threonine kinases protein kinase C, Byr2, and Raf-1, we propose that relief of autorepression and activation at the plasma membrane is an evolutionarily conserved mechanism of kinase regulation.
Figures


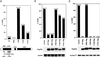
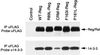


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

