Antiproliferative mechanism of action of cryptophycin-52: kinetic stabilization of microtubule dynamics by high-affinity binding to microtubule ends
- PMID: 9689077
- PMCID: PMC21335
- DOI: 10.1073/pnas.95.16.9313
Antiproliferative mechanism of action of cryptophycin-52: kinetic stabilization of microtubule dynamics by high-affinity binding to microtubule ends
Abstract
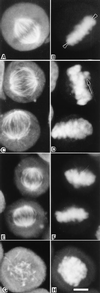
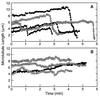
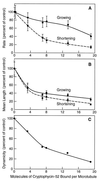
Cryptophycin-52 (LY355703) is a new synthetic member of the cryptophycin family of antimitotic antitumor agents that is currently undergoing clinical evaluation. At high concentrations (>/=10 times the IC50), cryptophycin-52 blocked HeLa cell proliferation at mitosis by depolymerizing spindle microtubules and disrupting chromosome organization. However, low concentrations of cryptophycin-52 inhibited cell proliferation at mitosis (IC50 = 11 pM) without significantly altering spindle microtubule mass or organization. Cryptophycin-52 appears to be the most potent suppressor of microtubule dynamics found thus far. It suppressed the dynamic instability behavior of individual microtubules in vitro (IC50 = 20 nM), reducing the rate and extent of shortening and growing without significantly reducing polymer mass or mean microtubule length. Using [3H]cryptophycin-52, we found that the compound bound to microtubule ends in vitro with high affinity (Kd, 47 nM, maximum of approximately 19.5 cryptophycin-52 molecules per microtubule). By analyzing the effects of cryptophycin-52 on dynamics in relation to its binding to microtubules, we determined that approximately 5-6 molecules of cryptophycin-52 bound to a microtubule were sufficient to decrease dynamicity by 50%. Cryptophycin-52 became concentrated in cells 730-fold, and the resulting intracellular cryptophycin-52 concentration was similar to that required to stabilize microtubule dynamics in vitro. The data suggest that cryptophycin-52 potently perturbs kinetic events at microtubule ends that are required for microtubule function during mitosis and that it acts by forming a reversible cryptophycin-52-tubulin stabilizing cap at microtubule ends.
Figures
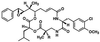

References
-
- Wilson L, Jordan M A. Chem Biol. 1995;2:569–573. - PubMed
-
- Wilson L, Jordan M A. In: Microtubules. Hyams J S, Lloyd C W, editors. New York: Wiley; 1994. pp. 59–83.
-
- Hamel E. In: Microtubule Proteins. Avila J, editor. Boca Raton, FL: CRC; 1990. pp. 89–191.
-
- Jordan M A, Thrower D, Wilson L. Cancer Res. 1991;51:2212–2222. - PubMed
-
- Jordan M A, Thrower D, Wilson L. J Cell Sci. 1992;102:401–416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

