A role for the actin-bundling protein L-plastin in the regulation of leukocyte integrin function
- PMID: 9689080
- PMCID: PMC21338
- DOI: 10.1073/pnas.95.16.9331
A role for the actin-bundling protein L-plastin in the regulation of leukocyte integrin function
Abstract
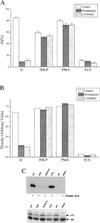
Regulation of leukocyte integrin avidity is a crucial aspect of inflammation and immunity. The actin cytoskeleton has an important role in the regulation of integrin function, but the cytoskeletal proteins involved are largely unknown. Because inflammatory stimuli that activate integrin-mediated adhesion in human polymorphonuclear neutrophils (PMN) and monocytes cause phosphorylation of the actin-bundling protein L-plastin, we tested whether L-plastin phosphorylation was involved in integrin activation. L-plastin-derived peptides that included the phosphorylation site (Ser-5) rapidly induced leukocyte integrin-mediated adhesion when introduced into the cytosol of freshly isolated primary human PMN and monocytes. Substitution of Ala for Ser-5 abolished the ability of the peptide to induce adhesion. Peptide-induced adhesion was sensitive to pharmacologic inhibition of phosphoinositol 3-kinase and protein kinase C, but adhesion induced by a peptide containing a phosphoserine at position 5 was insensitive to inhibition. These data establish a novel role for L-plastin in the regulation of leukocyte adhesion and suggest that many signaling events implicated in integrin regulation act via induction of L-plastin phosphorylation.
Figures
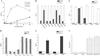

References
-
- Berton G, Yan S R, Fumagalli L, Lowell C A. Int J Clin Lab Res. 1996;26:160–177. - PubMed
-
- Diamond M S, Springer T A. Curr Biol. 1994;4:506–517. - PubMed
-
- Wright S D, Meyer B C. J Immunol. 1986;136:1758–1764. - PubMed
-
- Jones S L, Knaus U G, Bokoch G M, Brown E J. J Biol Chem. 1998;273:10556–10566. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

