Mineralocorticoid receptor knockout mice: pathophysiology of Na+ metabolism
- PMID: 9689096
- PMCID: PMC21354
- DOI: 10.1073/pnas.95.16.9424
Mineralocorticoid receptor knockout mice: pathophysiology of Na+ metabolism
Abstract
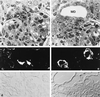
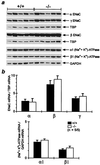
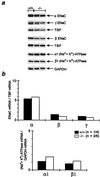
Mineralocorticoid receptor (MR)-deficient mice were generated by gene targeting. These animals had a normal prenatal development. During the first week of life, MR-deficient (-/-) mice developed symptoms of pseudohypoaldosteronism. They finally lost weight and eventually died at around day 10 after birth from dehydration by renal sodium and water loss. At day 8, -/- mice showed hyperkalemia, hyponatremia, and a strong increase in renin, angiotensin II, and aldosterone plasma concentrations. Methods were established to measure renal clearance and colonic transepithelial Na+ reabsorption in 8-day-old mice in vivo. The fractional renal Na+ excretion was elevated >8-fold. The glomerular filtration rate in -/- mice was not different from controls. The effect of amiloride on renal Na+ excretion and colonic transepithelial voltage reflects the function of amiloide-sensitive epithelial Na+ channels (ENaC). In -/- mice, it was reduced to 24% in the kidney and to 16% in the colon. There was, however, still significant residual ENaC-mediated Na+ reabsorption in both epithelia. RNase protection analysis of the subunits of ENaC and (Na++ K+)-ATPase did not reveal a decrease in -/- mice. The present data indicate that MR-deficient neonates die because they are not able to compensate renal Na+ loss. Regulation of Na+ reabsorption via MR is not achieved by transcriptional control of ENaC and (Na+ + K+)-ATPase in RNA abundance but by transcriptional control of other as yet unidentified genes. MR knockout mice will be a suitable tool for the search of these genes.
Figures

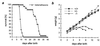
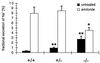
References
-
- Arriza J L, Weinberger C, Cerelli G, Glaser T M, Handelin B L, Housman D E, Evans R M. Science. 1987;237:268–275. - PubMed
-
- Funder J W. J Steroid Biochem Mol Biol. 1992;43:389–394. - PubMed
-
- Zennaro M C, Farman N, Bonvalet J P, Lombes M. J Clin Endocrinol Metab. 1997;82:1345–1352. - PubMed
-
- Arriza J L, Simerly R B, Swanson L W, Evans R M. Neuron. 1988;1:887–900. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

