A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis
- PMID: 9689113
- PMCID: PMC21371
- DOI: 10.1073/pnas.95.16.9524
A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis
Abstract
von Willebrand factor (vWf) deficiency causes severe von Willebrand disease in humans. We generated a mouse model for this disease by using gene targeting. vWf-deficient mice appeared normal at birth; they were viable and fertile. Neither vWf nor vWf propolypeptide (von Willebrand antigen II) were detectable in plasma, platelets, or endothelial cells of the homozygous mutant mice. The mutant mice exhibited defects in hemostasis with a highly prolonged bleeding time and spontaneous bleeding events in approximately 10% of neonates. As in the human disease, the factor VIII level in these mice was reduced strongly as a result of the lack of protection provided by vWf. Defective thrombosis in mutant mice was also evident in an in vivo model of vascular injury. In this model, the exteriorized mesentery was superfused with ferric chloride and the accumulation of fluorescently labeled platelets was observed by intravital microscopy. We conclude that these mice very closely mimic severe human von Willebrand disease and will be very useful for investigating the role of vWf in normal physiology and in disease models.
Figures
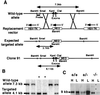
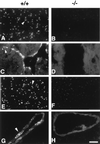
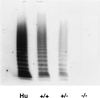



References
-
- Ewenstein B M. Annu Rev Med. 1997;48:525–542. - PubMed
-
- Andrews R K, Lopez J A, Berndt M C. Int J Biochem Cell Biol. 1997;29:91–105. - PubMed
-
- Bowie E J, Owen C A. In: Coagulation and Bleeding Disorders. Zimmerman T S, Ruggeri Z M, editors. New York: Dekker; 1989. pp. 305–324.
-
- Wu Q Y, Drouet L, Carrier J L, Rothchild C, Berard M, Rouault C, Caen J-P, Meyer D. Arteriosclerosis. 1987;7:47–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

