Cysteine and disulfide scanning reveals two amphiphilic helices in the linker region of the aspartate chemoreceptor
- PMID: 9692965
- PMCID: PMC2899697
- DOI: 10.1021/bi980607g
Cysteine and disulfide scanning reveals two amphiphilic helices in the linker region of the aspartate chemoreceptor
Abstract
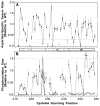
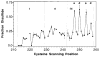
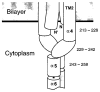
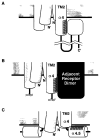
The transmembrane aspartate receptor of E. coli and S. typhimurium mediates cellular chemotaxis toward aspartate by regulating the activity of the cytoplasmic histidine kinase, CheA. Ligand binding results in transduction of a conformational signal through the membrane to the cytoplasmic domain where both kinase regulation and adaptation occur. Of particular interest is the linker region, E213 to Q258, which connects and transduces the conformational signal between the cytoplasmic end of the transmembrane signaling helix (alpha 4/TM2) and the major methylation helix of the cytoplasmic domain (alpha 6). This linker is crucial for stable folding and function of the homodimeric receptor. The present study uses cysteine and disulfide scanning mutagenesis to investigate the secondary structure and packing surfaces within the linker region. Chemical reactivity assays reveal that the linker consists of three distinct subdomains: two alpha-helices termed alpha 4 and alpha 5 and, between them, an ordered region of undetermined secondary structure. When cysteine is scanned through the helices, characteristic repeating patterns of solvent exposure and burial are observed. Activity assays, both in vivo and in vitro, indicate that each helix possesses a buried packing face that is crucial for proper receptor function. The interhelical subdomain is at least partially buried and is also crucial for proper receptor function. Disulfide scanning places helix alpha 4 distal to the central axis of the homodimer, while helix alpha 5 is found to lie at the subunit interface. Finally, sequence alignments suggest that all three linker subdomains are highly conserved among the large subfamily of histidine kinase-coupled sensory receptors that possess methylation sites for use in covalent adaptation.
Figures





References
-
- Wurgler-Murphy SM, Saito H. Trends Biochem Sci. 1997;22:172. - PubMed
-
- Stock AM, Mowbray SL. Curr Opin Struct Biol. 1995;5:744. - PubMed
-
- Stock JB, Surette MG. In: Escherichia coli and Salmonella Cellular and Molecular Biology. Neidhardt FC, editor. ASM Press; Washington, DC: 1996. pp. 1103–1129.
-
- Blair DF. Annu Rev Microbiol. 1995;49:489. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

