IkappaB is a substrate for a selective pathway of lysosomal proteolysis
- PMID: 9693362
- PMCID: PMC25451
- DOI: 10.1091/mbc.9.8.1995
IkappaB is a substrate for a selective pathway of lysosomal proteolysis
Abstract
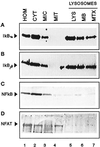
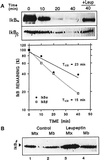
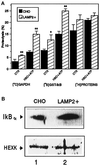
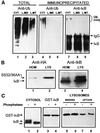
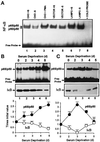
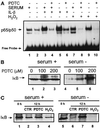
In lysosomes isolated from rat liver and spleen, a percentage of the intracellular inhibitor of the nuclear factor kappa B (IkappaB) can be detected in the lysosomal matrix where it is rapidly degraded. Levels of IkappaB are significantly higher in a lysosomal subpopulation that is active in the direct uptake of specific cytosolic proteins. IkappaB is directly transported into isolated lysosomes in a process that requires binding of IkappaB to the heat shock protein of 73 kDa (hsc73), the cytosolic molecular chaperone involved in this pathway, and to the lysosomal glycoprotein of 96 kDa (lgp96), the receptor protein in the lysosomal membrane. Other substrates for this degradation pathway competitively inhibit IkappaB uptake by lysosomes. Ubiquitination and phosphorylation of IkappaB are not required for its targeting to lysosomes. The lysosomal degradation of IkappaB is activated under conditions of nutrient deprivation. Thus, the half-life of a long-lived pool of IkappaB is 4.4 d in serum-supplemented Chinese hamster ovary cells but only 0.9 d in serum-deprived Chinese hamster ovary cells. This increase in IkappaB degradation can be completely blocked by lysosomal inhibitors. In Chinese hamster ovary cells exhibiting an increased activity of the hsc73-mediated lysosomal degradation pathway due to overexpression of lamp2, the human form of lgp96, the degradation of IkappaB is increased. There are both short- and long-lived pools of IkappaB, and it is the long-lived pool that is subjected to the selective lysosomal degradation pathway. In the presence of antioxidants, the half-life of the long-lived pool of IkappaB is significantly increased. Thus, the production of intracellular reactive oxygen species during serum starvation may be one of the mechanisms mediating IkappaB degradation in lysosomes. This selective pathway of lysosomal degradation of IkappaB is physiologically important since prolonged serum deprivation results in an increase in the nuclear activity of nuclear factor kappa B. In addition, the response of nuclear factor kappa B to several stimuli increases when this lysosomal pathway of proteolysis is activated.
Figures





References
-
- Adra CN, Zhu S, Ko J-L, et al. LAPTM5: a novel lysosomal-associated multispanning membrane protein preferentially expressed in hematopoietic cells. Genomics. 1996;35:328–337. - PubMed
-
- Aniento F, Roche E, Cuervo AM, Knecht E. Uptake and degradation of glyceraldehyde-3-phosphate dehydrogenase by rat liver lysosomes. J Biol Chem. 1993;268:10463–1070. - PubMed
-
- Aniento F, Papavassiliou AG, Knecht E, Roche E. Selective uptake and degradation of c-fos and v-fos by rat liver lysosomes. FEBS Lett. 1996;390:47–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

