Mutational effect of fission yeast polalpha on cell cycle events
- PMID: 9693370
- PMCID: PMC25465
- DOI: 10.1091/mbc.9.8.2107
Mutational effect of fission yeast polalpha on cell cycle events
Abstract
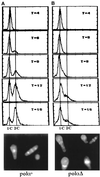
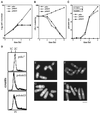
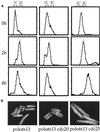
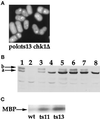
Polalpha is the principal DNA polymerase for initiation of DNA replication and also functions in postinitiation DNA synthesis. In this study, we investigated the cell cycle responses induced by mutations in polalpha+. Germinating spores carrying either a deletion of polalpha+ (polalphaDelta) or a structurally intact but catalytically dead polalpha mutation proceed to inappropriate mitosis with no DNA synthesis. This suggests that the catalytic function, and not the physical presence of Polalpha, is required to generate the signal that prevents the cells from entering mitosis prematurely. Cells with a polalphats allele arrest the cell cycle near the hydroxyurea arrest point, but, surprisingly, polalphats in cdc20 (polepsilon mutant) background arrested with a cdc phenoytpe, not a polalphats-like phenotype. At 25 degrees C, replication perturbation caused by polalphats alleles induces Cds1 kinase activity and requires the checkpoint Rads, Cds1, and Rqh1, but not Chk1, to maintain cell viability. At 36 degrees C, replication disruption caused by polalphats alleles induces the phosphorylation of Chk1; however, mutant cells arrest with heterogeneous cell sizes with a population of the cells entering aberrant mitosis. Together, our results indicate that the initiation DNA structure synthesized by Polalpha is required to bring about the S phase to mitosis checkpoint, whereas replication defects of different severity caused by polalphats mutations induce differential downstream kinase responses.
Figures


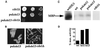
References
-
- Aparicio OM, Weinstein DM, Bell SP. Components and dynamics of DNA replication complexes in S. cerevisiae: redistribution of MCM proteins and Cdc45p during S phase. Cell. 1997;91:59–69. - PubMed
-
- Boeke JD, Trueheart J, Natsoulis G, Fink GR. 5-Fluoroorotic acid a selective agent in yeast molecular genetics. Methods Enzymol. 1987;154:164–175. - PubMed
-
- Brun C, Dubey DD, Huberman JA. pDblet, a stable autonomously replicating shuttle vector for Schizosaccharomyces pombe. Gene. 1995;164:173–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

