The Saccharomyces cerevisiae prenylcysteine carboxyl methyltransferase Ste14p is in the endoplasmic reticulum membrane
- PMID: 9693378
- PMCID: PMC25475
- DOI: 10.1091/mbc.9.8.2231
The Saccharomyces cerevisiae prenylcysteine carboxyl methyltransferase Ste14p is in the endoplasmic reticulum membrane
Abstract
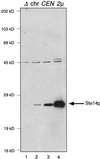
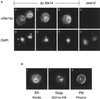
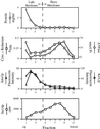
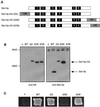
Eukaryotic proteins containing a C-terminal CAAX motif undergo a series of posttranslational CAAX-processing events that include isoprenylation, C-terminal proteolytic cleavage, and carboxyl methylation. We demonstrated previously that the STE14 gene product of Saccharomyces cerevisiae mediates the carboxyl methylation step of CAAX processing in yeast. In this study, we have investigated the subcellular localization of Ste14p, a predicted membrane-spanning protein, using a polyclonal antibody generated against the C terminus of Ste14p and an in vitro methyltransferase assay. We demonstrate by immunofluorescence and subcellular fractionation that Ste14p and its associated activity are localized to the endoplasmic reticulum (ER) membrane of yeast. In addition, other studies from our laboratory have shown that the CAAX proteases are also ER membrane proteins. Together these results indicate that the intracellular site of CAAX protein processing is the ER membrane, presumably on its cytosolic face. Interestingly, the insertion of a hemagglutinin epitope tag at the N terminus, at the C terminus, or at an internal site disrupts the ER localization of Ste14p and results in its mislocalization, apparently to the Golgi. We have also expressed the Ste14p homologue from Schizosaccharomyces pombe, mam4p, in S. cerevisiae and have shown that mam4p complements a Deltaste14 mutant. This finding, plus additional recent examples of cross-species complementation, indicates that the CAAX methyltransferase family consists of functional homologues.
Figures


References
-
- Backlund PS. Post-translational processing of RhoA. J Biol Chem. 1997;272:33175–33180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

