A histone deacetylase corepressor complex regulates the Notch signal transduction pathway
- PMID: 9694793
- PMCID: PMC317043
- DOI: 10.1101/gad.12.15.2269
A histone deacetylase corepressor complex regulates the Notch signal transduction pathway
Abstract
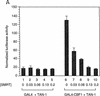
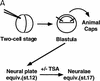
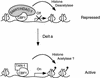
The Delta-Notch signal transduction pathway has widespread roles in animal development in which it appears to control cell fate. CBF1/RBP-Jkappa, the mammalian homolog of Drosophila Suppressor of Hairless [Su(H)], switches from a transcriptional repressor to an activator upon Notch activation. The mechanism whereby Notch regulates this switch is not clear. In this report we show that prior to induction CBF1/RBP-Jkappa interacts with a corepressor complex containing SMRT (silencing mediator of retinoid and thyroid hormone receptors) and the histone deacetylase HDAC-1. This complex binds via the CBF1 repression domain, and mutants defective in repression fail to interact with the complex. Activation by Notch disrupts the formation of the repressor complex, thus establishing a molecular basis for the Notch switch. Finally, ESR-1, a Xenopus gene activated by Notch and X-Su(H), is induced in animal caps treated with TSA, an inhibitor of HDAC-1. The functional role for the SMRT/HDAC-1 complex in CBF1/RBP-Jkappa regulation reveals a novel genetic switch in which extracellular ligands control the status of critical nuclear cofactor complexes.
Figures
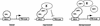
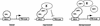

















References
-
- Alland L, Muhle R, Hou H, Potes JJ, Chin L, Schreiber-Agus N, DePinho RA. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature. 1997;387:49–55. - PubMed
-
- Amakawa R, Jing W, Ozawa K. Human Jk recombination signal binding protein gene (IGKJRB): Comparison with its mouse homologue. Genomics. 1993;17:306–315. - PubMed
-
- Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268:225–232. - PubMed
-
- Ayer DE, Lawrence QA. Mad-Max transcriptional repression is mediated by ternary complex formation with mammalian homologs of yeast repressor Sin3. Cell. 1995;80:767–776. - PubMed
-
- Bailey AM, Posakony JW. Suppressor of hairless directly activates transcription of enhancer of split complex genes in response to Notch receptor activity. Genes & Dev. 1995;9:2609–2622. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
