The ROTUNDIFOLIA3 gene of Arabidopsis thaliana encodes a new member of the cytochrome P-450 family that is required for the regulated polar elongation of leaf cells
- PMID: 9694802
- PMCID: PMC317051
- DOI: 10.1101/gad.12.15.2381
The ROTUNDIFOLIA3 gene of Arabidopsis thaliana encodes a new member of the cytochrome P-450 family that is required for the regulated polar elongation of leaf cells
Abstract
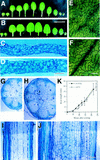
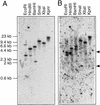
The polarized processes of cell elongation play a crucial role in morphogenesis of higher plants. We reported previously that the rotundifolia3 (rot3) mutant of Arabidopsis has a defect in the polar elongation of leaf cells. In the present study, we isolated two additional alleles with mutations in the ROT3 gene. The ROT3 gene was cloned by a T-DNA-tagging method and isolation of the gene was confirmed by a molecular analysis of three rot3 mutant alleles obtained from different mutagenesis. The ROT3 gene encodes a cytochrome P-450 (CYP90C1) with domains homologous to regions of steroid hydroxylases of animals and plants. Expression of the ROT3 gene was detected in all major plant organs. Especially, higher expression was detected in the tissues that had high activity of cell division. We confirmed that the ROT3 gene controls polar elongation specifically in leaf cells by an analysis of three rot3 mutants obtained from different mutagenesis experiments. Our results imply that the ROT3 protein is a member of a new class of cytochrome P-450 encoding putative steroid hydroxylases, which is required for the regulated polar elongation of cells in leaves of Arabidopsis.
Figures






References
-
- Chen C-D, Kemper B. Different structural requirements at specific proline residue positions in the conserved proline-rich region of cytochrome P450 2C2. J Biol Chem. 1996;271:28607–28611. - PubMed
-
- Chen C-D, Doray B, Kemper B. A conserved proline-rich sequence between the N-terminal signal-anchor and catalytic domains is required for assembly of functional cytochrome P450 2C2. Arch Biochem Biophys. 1998;350:233–238. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
