Insertion mutation of the form I cbbL gene encoding ribulose bisphosphate carboxylase/oxygenase (RuBisCO) in Thiobacillus neapolitanus results in expression of form II RuBisCO, loss of carboxysomes, and an increased CO2 requirement for growth
- PMID: 9696760
- PMCID: PMC107408
- DOI: 10.1128/JB.180.16.4133-4139.1998
Insertion mutation of the form I cbbL gene encoding ribulose bisphosphate carboxylase/oxygenase (RuBisCO) in Thiobacillus neapolitanus results in expression of form II RuBisCO, loss of carboxysomes, and an increased CO2 requirement for growth
Abstract
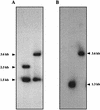
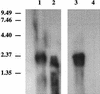
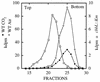
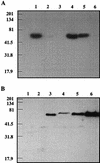
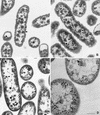
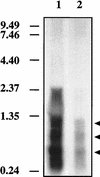
It has been previously established that Thiobacillus neapolitanus fixes CO2 by using a form I ribulose bisphosphate carboxylase/oxygenase (RuBisCO), that much of the enzyme is sequestered into carboxysomes, and that the genes for the enzyme, cbbL and cbbS, are part of a putative carboxysome operon. In the present study, cbbL and cbbS were cloned and sequenced. Analysis of RNA showed that cbbL and cbbS are cotranscribed on a message approximately 2,000 nucleotides in size. The insertion of a kanamycin resistance cartridge into cbbL resulted in a premature termination of transcription; a polar mutant was generated. The mutant is able to fix CO2, but requires a CO2 supplement for growth. Separation of cellular proteins from both the wild type and the mutant on sucrose gradients and subsequent analysis of the RuBisCO activity in the collected fractions showed that the mutant assimilates CO2 by using a form II RuBisCO. This was confirmed by immunoblot analysis using antibodies raised against form I and form II RuBisCOs. The mutant does not possess carboxysomes. Smaller, empty inclusions are present, but biochemical analysis indicates that if they are carboxysome related, they are not functional, i.e., do not contain RuBisCO. Northern analysis showed that some of the shell components of the carboxysome are produced, which may explain the presence of these inclusions in the mutant.
Figures

References
-
- Beudeker R F, Codd G A, Kuenen J G. Quantification and intracellular distribution of ribulose-1,5-bisphosphate carboxylase in Thiobacillus neapolitanus, as related to possible functions of carboxysomes. Arch Microbiol. 1981;129:361–367.
-
- Cannon G C, Shively J M. Characterization of a homogenous preparation of carboxysomes from Thiobacillus neapolitanus. Arch Microbiol. 1983;134:52–59.
-
- Chung S Y, Yaguchi T, Nishihara H, Igarashi Y, Kodama T. Purification of form L2 RubisCO from a marine obligately autotrophic hydrogen-oxidizing bacterium, Hydrogenovibrio marinus strain MH-110. FEMS Microbiol Lett. 1993;109:49–53. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

