Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry
- PMID: 9696799
- PMCID: PMC109927
- DOI: 10.1128/JVI.72.9.7064-7074.1998
Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry
Abstract
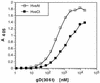
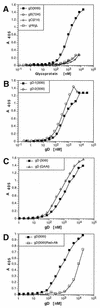
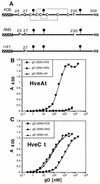
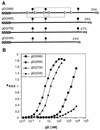
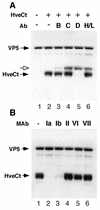
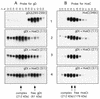
Several cell membrane proteins have been identified as herpes simplex virus (HSV) entry mediators (Hve). HveA (formerly HVEM) is a member of the tumor necrosis factor receptor family, whereas the poliovirus receptor-related proteins 1 and 2 (PRR1 and PRR2, renamed HveC and HveB) belong to the immunoglobulin superfamily. Here we show that a truncated form of HveC directly binds to HSV glycoprotein D (gD) in solution and at the surface of virions. This interaction is dependent on the native conformation of gD but independent of its N-linked glycosylation. Complex formation between soluble gD and HveC appears to involve one or two gD molecules for one HveC protein. Since HveA also mediates HSV entry by interacting with gD, we compared both structurally unrelated receptors for their binding to gD. Analyses of several gD variants indicated that structure and accessibility of the N-terminal domain of gD, essential for HveA binding, was not necessary for HveC interaction. Mutations in functional regions II, III, and IV of gD had similar effects on binding to either HveC or HveA. Competition assays with neutralizing anti-gD monoclonal antibodies (MAbs) showed that MAbs from group Ib prevented HveC and HveA binding to virions. However, group Ia MAbs blocked HveC but not HveA binding, and conversely, group VII MAbs blocked HveA but not HveC binding. Thus, we propose that HSV entry can be mediated by two structurally unrelated gD receptors through related but not identical binding with gD.
Figures

References
-
- Aoki J, Koike S, Asou H, Ise I, Suwa H, Tanaka T, Miyasaka M, Nomoto A. Mouse homolog of poliovirus receptor-related gene 2 product, mPRR2, mediates homophilic cell aggregation. Exp Cell Res. 1997;235:374–384. - PubMed
-
- Buckmaster E A, Gompels U, Minson A. Characterisation and physical mapping of an HSV-1 glycoprotein of approximately 115 × 10(3) molecular weight. Virology. 1984;139:408–413. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

