Control of murine cytomegalovirus in the lungs: relative but not absolute immunodominance of the immediate-early 1 nonapeptide during the antiviral cytolytic T-lymphocyte response in pulmonary infiltrates
- PMID: 9696814
- PMCID: PMC109942
- DOI: 10.1128/JVI.72.9.7201-7212.1998
Control of murine cytomegalovirus in the lungs: relative but not absolute immunodominance of the immediate-early 1 nonapeptide during the antiviral cytolytic T-lymphocyte response in pulmonary infiltrates
Abstract
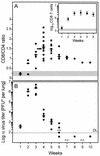
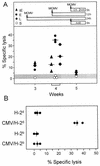
The lungs are a major organ site of cytomegalovirus (CMV) infection, pathogenesis, and latency. Interstitial CMV pneumonia represents a critical manifestation of CMV disease, in particular in recipients of bone marrow transplantation (BMT). We have employed a murine model for studying the immune response to CMV in the lungs in the specific scenario of immune reconstitution after syngeneic BMT. Control of pulmonary infection was associated with a vigorous infiltration of the lungs, which was characterized by a preferential recruitment and massive expansion of the CD8 subset of alpha/beta T cells. The infiltrate provided a microenvironment in which the CD8 T cells differentiated into mature effector cells, that is, into functionally active cytolytic T lymphocytes (CTL). This gave us the opportunity for an ex vivo testing of the antigen specificities of CTL present at a relevant organ site of viral pathogenesis. The contribution of the previously identified immediate-early 1 (IE1) nonapeptide of murine CMV was evaluated by comparison with the CD3epsilon-redirected cytolytic activity used as a measure of the overall CTL response in the lungs. The IE1 peptide was detected by pulmonary CTL, but it accounted for a minor part of the response. Interestingly, no additional viral or virus-induced antigenic peptides were detectable among naturally processed peptides derived from infected lungs, even though infected fibroblasts were recognized in a major histocompatibility complex-restricted manner. We conclude that the antiviral pulmonary immune response is a collaborative function that involves many antigenic peptides, among which the IE1 peptide is immunodominant in a relative sense.
Figures






References
-
- Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Phenotype analysis of antigen-specific T lymphocytes. Science (Washington, DC) 1996;274:94–96. - PubMed
-
- Azuma M, Cayabyab M, Phillips J H, Lanier L L. Requirements for CD28-dependent T cell-mediated cytotoxicity. J Immunol. 1993;150:2091–2101. - PubMed
-
- Bancroft G J, Shellam G R, Chalmer J E. Genetic influence on the augmentation of natural killer (NK) cells during murine cytomegalovirus infection: correlation with patterns of resistance. J Immunol. 1981;126:988–994. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

