Heparin-like structures on respiratory syncytial virus are involved in its infectivity in vitro
- PMID: 9696816
- PMCID: PMC109944
- DOI: 10.1128/JVI.72.9.7221-7227.1998
Heparin-like structures on respiratory syncytial virus are involved in its infectivity in vitro
Abstract
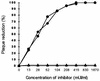
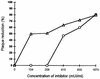
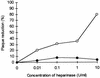
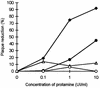
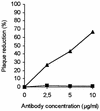
Addition of heparin to the virus culture inhibited syncytial plaque formation due to respiratory syncytial virus (RSV). Moreover, pretreatment of the virus with heparinase or an inhibitor of heparin, protamine, greatly reduced virus infectivity. Two anti-heparan sulfate antibodies stained RSV-infected cells, but not noninfected cells, by immunofluorescence. One of the antibodies was capable of neutralizing RSV infection in vitro. These results prove that heparin-like structures identified on RSV play a major role in early stages of infection. The RSV G protein is the attachment protein. Both anti-heparan sulfate antibodies specifically bound to this protein. Enzymatic digestion of polysaccharides in the G protein reduced the binding, which indicates that heparin-like structures are on the G protein. Such oligosaccharides may therefore participate in the attachment of the virus.
Figures

Similar articles
-
The fusion glycoprotein of human respiratory syncytial virus facilitates virus attachment and infectivity via an interaction with cellular heparan sulfate.J Virol. 2000 Jul;74(14):6442-7. doi: 10.1128/jvi.74.14.6442-6447.2000. J Virol. 2000. PMID: 10864656 Free PMC article.
-
Iduronic acid-containing glycosaminoglycans on target cells are required for efficient respiratory syncytial virus infection.Virology. 2000 Jun 5;271(2):264-75. doi: 10.1006/viro.2000.0293. Virology. 2000. PMID: 10860881
-
Heparin-dependent attachment of respiratory syncytial virus (RSV) to host cells.Arch Virol. 1997;142(6):1247-54. doi: 10.1007/s007050050156. Arch Virol. 1997. PMID: 9229012
-
Respiratory syncytial virus with the fusion protein as its only viral glycoprotein is less dependent on cellular glycosaminoglycans for attachment than complete virus.Virology. 2002 Mar 15;294(2):296-304. doi: 10.1006/viro.2001.1340. Virology. 2002. PMID: 12009871
-
Importance of Heparan Sulfate Proteoglycans in Pancreatic Islets and β-Cells.Int J Mol Sci. 2022 Oct 11;23(20):12082. doi: 10.3390/ijms232012082. Int J Mol Sci. 2022. PMID: 36292936 Free PMC article. Review.
Cited by
-
The Potential Role of Coagulation Factor Xa in the Pathophysiology of COVID-19: A Role for Anticoagulants as Multimodal Therapeutic Agents.TH Open. 2020 Oct 7;4(4):e288-e299. doi: 10.1055/s-0040-1718415. eCollection 2020 Oct. TH Open. 2020. PMID: 33043235 Free PMC article. Review.
-
In vitro modeling of respiratory syncytial virus infection of pediatric bronchial epithelium, the primary target of infection in vivo.Proc Natl Acad Sci U S A. 2012 Mar 27;109(13):5040-5. doi: 10.1073/pnas.1110203109. Epub 2012 Mar 12. Proc Natl Acad Sci U S A. 2012. PMID: 22411804 Free PMC article.
-
Receptors for Respiratory Syncytial Virus Infection and Host Factors Regulating the Life Cycle of Respiratory Syncytial Virus.Front Cell Infect Microbiol. 2022 Feb 25;12:858629. doi: 10.3389/fcimb.2022.858629. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35281439 Free PMC article. Review.
-
Unity in diversity: shared mechanism of entry among paramyxoviruses.Prog Mol Biol Transl Sci. 2015;129:1-32. doi: 10.1016/bs.pmbts.2014.10.001. Epub 2014 Dec 1. Prog Mol Biol Transl Sci. 2015. PMID: 25595799 Free PMC article. Review.
-
Identification of linear heparin-binding peptides derived from human respiratory syncytial virus fusion glycoprotein that inhibit infectivity.J Virol. 2007 Jan;81(1):261-71. doi: 10.1128/JVI.01226-06. Epub 2006 Oct 18. J Virol. 2007. PMID: 17050595 Free PMC article.
References
-
- Anderson K, King A M Q, Lerch R A, Wertz G W. Polylactosaminoglycan modification of the respiratory syncytial virus small hydrophobic (SH) protein: a conserved feature among human and bovine respiratory syncytial viruses. Virology. 1992;191:417–430. - PubMed
-
- Apostolopoulos V, McKenzie I F C. Cellular mucins: targets for immunotherapy. Crit Rev Immunol. 1994;14:293–309. - PubMed
-
- Bourgeois C, Corvaisier C, Bour J B, Kohli E, Pothier P. Use of synthetic peptides to locate neutralizing antigenic domains on the fusion protein of respiratory syncytial virus. J Gen Virol. 1991;72:1051–1058. - PubMed
-
- Choppin P W, Scheid A. The role of viral glycoproteins in adsorption, penetration and pathogenicity of viruses. Rev Infect Dis. 1980;2:40–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

