Purified, soluble recombinant mouse hepatitis virus receptor, Bgp1(b), and Bgp2 murine coronavirus receptors differ in mouse hepatitis virus binding and neutralizing activities
- PMID: 9696818
- PMCID: PMC109946
- DOI: 10.1128/JVI.72.9.7237-7244.1998
Purified, soluble recombinant mouse hepatitis virus receptor, Bgp1(b), and Bgp2 murine coronavirus receptors differ in mouse hepatitis virus binding and neutralizing activities
Abstract
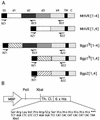
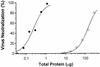
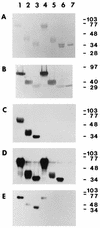
Mouse hepatitis virus receptor (MHVR) is a murine biliary glycoprotein (Bgp1(a)). Purified, soluble MHVR expressed from a recombinant vaccinia virus neutralized the infectivity of the A59 strain of mouse hepatitis virus (MHV-A59) in a concentration-dependent manner. Several anchored murine Bgps in addition to MHVR can also function as MHV-A59 receptors when expressed at high levels in nonmurine cells. To investigate the interactions of these alternative MHVR glycoproteins with MHV, we expressed and purified to apparent homogeneity the extracellular domains of several murine Bgps as soluble, six-histidine-tagged glycoproteins, using a baculovirus expression system. These include MHVR isoforms containing four or two extracellular domains and the corresponding Bgp1(b) glycoproteins from MHV-resistant SJL/J mice, as well as Bgp2 and truncation mutants of MHVR and Bgp1(b) comprised of the first two immunoglobulin-like domains. The soluble four-domain MHVR glycoprotein (sMHVR[1-4]) had fourfold more MHV-A59 neutralizing activity than the corresponding soluble Bgp1(b) (sBgp1(b)) glycoprotein and at least 1,000-fold more neutralizing activity than sBgp2. Although virus binds to the N-terminal domain (domain 1), soluble truncation mutants of MHVR and Bgp1(b) containing only domains 1 and 2 bound virus poorly and had 10- and 300-fold less MHV-A59 neutralizing activity than the corresponding four-domain glycoproteins. In contrast, the soluble MHVR glycoprotein containing domains 1 and 4 (sMHVR[1,4]) had as much neutralizing activity as the four-domain glycoprotein, sMHVR[1-4]. Thus, the virus neutralizing activity of MHVR domain 1 appears to be enhanced by domain 4. The sBgp1(b)[1-4] glycoprotein had 500-fold less neutralizing activity for MHV-JHM than for MHV-A59. Thus, MHV strains with differences in S-glycoprotein sequence, tissue tropism, and virulence can differ in the ability to utilize the various murine Bgps as receptors.
Figures


Similar articles
-
Neutralization of MHV-A59 by soluble recombinant receptor glycoproteins.Adv Exp Med Biol. 1998;440:3-9. doi: 10.1007/978-1-4615-5331-1_1. Adv Exp Med Biol. 1998. PMID: 9782258
-
The murine coronavirus mouse hepatitis virus strain A59 from persistently infected murine cells exhibits an extended host range.J Virol. 1997 Dec;71(12):9499-507. doi: 10.1128/JVI.71.12.9499-9507.1997. J Virol. 1997. PMID: 9371612 Free PMC article.
-
Mutational analysis of the virus and monoclonal antibody binding sites in MHVR, the cellular receptor of the murine coronavirus mouse hepatitis virus strain A59.J Virol. 1998 Mar;72(3):1941-8. doi: 10.1128/JVI.72.3.1941-1948.1998. J Virol. 1998. PMID: 9499047 Free PMC article.
-
Characterization of a new gene that encodes a functional MHV receptor and progress in the identification of the virus-binding site(s).Adv Exp Med Biol. 1995;380:345-50. doi: 10.1007/978-1-4615-1899-0_56. Adv Exp Med Biol. 1995. PMID: 8830505 Review.
-
Morphological analysis of mouse hepatitis virus A59-induced pathology with regard to viral receptor expression.Histol Histopathol. 1998 Jan;13(1):181-99. doi: 10.14670/HH-13.181. Histol Histopathol. 1998. PMID: 9476648 Review.
Cited by
-
The N-terminal region of the murine coronavirus spike glycoprotein is associated with the extended host range of viruses from persistently infected murine cells.J Virol. 2004 Sep;78(17):9073-83. doi: 10.1128/JVI.78.17.9073-9083.2004. J Virol. 2004. PMID: 15308703 Free PMC article.
-
CEACAM1 recognition by bacterial pathogens is species-specific.BMC Microbiol. 2010 Apr 20;10:117. doi: 10.1186/1471-2180-10-117. BMC Microbiol. 2010. PMID: 20406467 Free PMC article.
-
The N-terminal domain of the murine coronavirus spike glycoprotein determines the CEACAM1 receptor specificity of the virus strain.J Virol. 2003 Jan;77(2):841-50. doi: 10.1128/jvi.77.2.841-850.2003. J Virol. 2003. PMID: 12502800 Free PMC article.
-
Systematic assembly of a full-length infectious cDNA of mouse hepatitis virus strain A59.J Virol. 2002 Nov;76(21):11065-78. doi: 10.1128/jvi.76.21.11065-11078.2002. J Virol. 2002. PMID: 12368349 Free PMC article.
-
Amino acid substitutions and an insertion in the spike glycoprotein extend the host range of the murine coronavirus MHV-A59.Virology. 2004 Jul 1;324(2):510-24. doi: 10.1016/j.virol.2004.04.005. Virology. 2004. PMID: 15207636 Free PMC article.
References
-
- Asanaka M, Lai M M. Cell fusion studies identified multiple cellular factors involved in mouse hepatitis virus entry. Virology. 1993;197:732–741. - PubMed
-
- Banfield M J, King D J, Mountain A, Brady R L. VL:VH domain rotations in engineered antibodies: crystal structures of the Fab fragments from two murine antitumor antibodies and their engineered human constructs. Proteins. 1997;29:161–171. - PubMed
-
- Barthold S W. Mouse hepatitis virus: biology and epizootiology. In: Bhatt P N, Jacoby R O, Morse III H C, New A E, editors. Viral and mycoplasma infections of laboratory rodents. Effects on biomedical research. Orlando, Fla: Academic Press; 1986. pp. 571–601.
-
- Bates P A, Luo J, Sternberg M J. A predicted three-dimensional structure for the carcinoembryonic antigen (CEA) FEBS Lett. 1992;301:207–214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

