The disulfide-bonded structure of feline herpesvirus glycoprotein I
- PMID: 9696819
- PMCID: PMC109947
- DOI: 10.1128/JVI.72.9.7245-7254.1998
The disulfide-bonded structure of feline herpesvirus glycoprotein I
Abstract
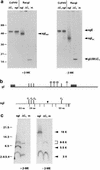
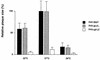
Alphaherpesvirus glycoproteins E and I (gE and gI, respectively) assemble into a hetero-oligomeric complex which promotes cell-to-cell transmission, a determining factor of virulence. Focusing on gI of feline herpesvirus (FHV), we examined the role of disulfide bonds during its biosynthesis, its interaction with gE, and gE-gI-mediated spread of the infection in vitro. The protein's disulfide linkage pattern was determined by single and pairwise substitutions for the four conserved cysteine residues in the ectodomain. The resulting mutants were coexpressed with gE in the vaccinia virus-based vTF7-3 system, and the formation and endoplasmic reticulum (ER)-to-Golgi transport of the hetero-oligomeric complex were monitored. The results were corroborated biochemically by performing an endoproteinase Lys-C digestion of a [35S]Cys-labeled secretory recombinant form of gI followed by tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of the peptides under reducing and nonreducing conditions. We found that (i) gI derivatives lacking Cys79 (C1) and/or Cys223 (C4) still assemble with gE into transport-competent complexes, (ii) mutant proteins lacking Cys91 (C2) and/or Cys102 (C3) bind to gE but are retained in the ER, (iii) radiolabeled endoproteinase Lys-C-generated peptide species containing C1 and C4 are linked through disulfide bonds, and (iv) peptides containing both C2 and C3 are not disulfide linked to any other peptide. From these findings emerges a model in which C1 and C4 as well as C2 and C3 form intramolecular disulfide bridges. Since the cysteines in the ectodomain have been conserved during alphaherpesvirus divergence, we postulate that the model applies for all gI proteins. Analysis of an FHV recombinant with a C1-->S substitution confirmed that the C1-C4 disulfide bond is not essential for the formation of a transport-competent gE-gI complex. The mutation affected the posttranslational modification of gI and caused a slight cold-sensitivity defect in the assembly or the intracellular transport of the gE-gI complex but did not affect plaque size. Thus, C1 and the C1-C4 bond are not essential for gE-gI-mediated cell-to-cell spread, at least not in vitro.
Figures







References
-
- Audonnet J C, Winslow J, Allen G, Paoletti E. Equine herpesvirus type 1 unique short fragment encodes glycoproteins with homology to herpes simplex virus type 1 gD, gI and gE. J Gen Virol. 1990;71:2969–2978. - PubMed
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley-Interscience; 1989.
-
- Balan P, Davis Poynter N, Bell S, Atkinson H, Browne H, Minson T. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoproteins gG, gE, gI or the putative gJ. J Gen Virol. 1994;75:1245–1258. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

