DNA vaccination affords significant protection against feline immunodeficiency virus infection without inducing detectable antiviral antibodies
- PMID: 9696827
- PMCID: PMC109955
- DOI: 10.1128/JVI.72.9.7310-7319.1998
DNA vaccination affords significant protection against feline immunodeficiency virus infection without inducing detectable antiviral antibodies
Erratum in
- J Virol 1998 Oct;72(10):8460
Abstract
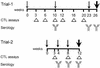
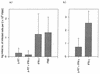
To test the potential of a multigene DNA vaccine against lentivirus infection, we generated a defective mutant provirus of feline immunodeficiency virus (FIV) with an in-frame deletion in pol (FIVDeltaRT). In a first experiment, FIVDeltaRT DNA was administered intramuscularly to 10 animals, half of which also received feline gamma interferon (IFN-gamma) DNA. The DNA was administered in four 100-microg doses at 0, 10, and 23 weeks. Immunization with FIVDeltaRT elicited cytotoxic T-cell (CTL) responses to FIV Gag and Env in the absence of a serological response. After challenge with homologous virus at week 26, all 10 of the control animals became seropositive and viremic but 4 of the 10 vaccinates remained seronegative and virus free. Furthermore, quantitative virus isolation and quantitative PCR analysis of viral DNA in peripheral blood mononuclear cells revealed significantly lower virus loads in the FIVDeltaRT vaccinates than in the controls. Immunization with FIVDeltaRT in conjunction with IFN-gamma gave the highest proportion of protected cats, with only two of five vaccinates showing evidence of infection following challenge. In a second experiment involving two groups (FIVDeltaRT plus IFN-gamma and IFN-gamma alone), the immunization schedule was reduced to 0, 4, and 8 weeks. Once again, CTL responses were seen prior to challenge in the absence of detectable antibodies. Two of five cats receiving the proviral DNA vaccine were protected against infection, with an overall reduction in virus load compared to the five infected controls. These findings demonstrate that DNA vaccination can elicit protection against lentivirus infection in the absence of a serological response and suggest the need to reconsider efficacy criteria for lentivirus vaccines.
Figures

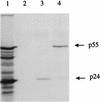
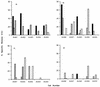
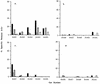
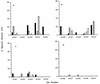
 ) or FIV Env (▨ and ▨) or no FIV proteins (infected with
wild-type vaccinia virus [□]). The results shown represent the mean
51Cr release after a 4-h incubation at 37°C for
triplicate cultures at an E/T ratio of 50:1. ND, not done.
) or FIV Env (▨ and ▨) or no FIV proteins (infected with
wild-type vaccinia virus [□]). The results shown represent the mean
51Cr release after a 4-h incubation at 37°C for
triplicate cultures at an E/T ratio of 50:1. ND, not done.
Similar articles
-
Vaccination of cats with attenuated feline immunodeficiency virus proviral DNA vaccine expressing gamma interferon.J Virol. 2007 Jan;81(2):465-73. doi: 10.1128/JVI.00815-06. Epub 2006 Nov 1. J Virol. 2007. PMID: 17079309 Free PMC article.
-
Protective immunity against feline immunodeficiency virus induced by inoculation with vif-deleted proviral DNA.Virology. 2000 Jul 20;273(1):67-79. doi: 10.1006/viro.2000.0395. Virology. 2000. PMID: 10891409
-
Protection against FIV challenge infection by genetic vaccination using minimalistic DNA constructs for FIV env gene and feline IL-12 expression.AIDS. 2000 Aug 18;14(12):1749-57. doi: 10.1097/00002030-200008180-00009. AIDS. 2000. PMID: 10985311
-
Feline immunodeficiency virus vaccine: implications for diagnostic testing and disease management.Biologicals. 2005 Dec;33(4):215-7. doi: 10.1016/j.biologicals.2005.08.004. Epub 2005 Oct 28. Biologicals. 2005. PMID: 16257536 Review.
-
Vaccination of cats with recombinant envelope glycoprotein of feline immunodeficiency virus: decreased viral load after challenge infection.AIDS Res Hum Retroviruses. 1996 Mar 20;12(5):431-3. doi: 10.1089/aid.1996.12.431. AIDS Res Hum Retroviruses. 1996. PMID: 8882327 Review. No abstract available.
Cited by
-
Effective induction of simian immunodeficiency virus-specific systemic and mucosal immune responses in primates by vaccination with proviral DNA producing intact but noninfectious virions.J Virol. 2000 Nov;74(22):10514-22. doi: 10.1128/jvi.74.22.10514-10522.2000. J Virol. 2000. PMID: 11044096 Free PMC article.
-
The Significance of Interferon-γ in HIV-1 Pathogenesis, Therapy, and Prophylaxis.Front Immunol. 2014 Jan 13;4:498. doi: 10.3389/fimmu.2013.00498. eCollection 2014 Jan 13. Front Immunol. 2014. PMID: 24454311 Free PMC article. Review.
-
An initial examination of the potential role of T-cell immunity in protection against feline immunodeficiency virus (FIV) infection.Vaccine. 2016 Mar 14;34(12):1480-8. doi: 10.1016/j.vaccine.2016.01.017. Epub 2016 Jan 21. Vaccine. 2016. PMID: 26802606 Free PMC article.
-
Longitudinal analysis of feline leukemia virus-specific cytotoxic T lymphocytes: correlation with recovery from infection.J Virol. 2002 Mar;76(5):2306-15. doi: 10.1128/jvi.76.5.2306-2315.2002. J Virol. 2002. PMID: 11836409 Free PMC article.
-
Vaccination of cats with attenuated feline immunodeficiency virus proviral DNA vaccine expressing gamma interferon.J Virol. 2007 Jan;81(2):465-73. doi: 10.1128/JVI.00815-06. Epub 2006 Nov 1. J Virol. 2007. PMID: 17079309 Free PMC article.
References
-
- Argyle D J, Smith K, McBride K, Fulton R, Onions D E. Nucleotide and predicted peptide sequence of feline interferon-gamma (IFN-gamma) DNA Sequence. 1995;5:169–171. - PubMed
-
- Avrameas A, Strosberg A D, Moraillon A, Sonigo P, Pancino G. Serological diagnosis of feline immunodeficiency virus (FIV) infection based on synthetic peptides from Env glycoproteins. Res Virol. 1993;144:209–218. - PubMed
-
- Baba T W, Jeong Y S, Penninck D, Bronson R, Greene M F, Ruprecht R M. Pathogenicity of live attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267:1820–1825. - PubMed
-
- Boyer J D, Ugen K E, Wang B, Agadjanyan M, Gilbert L, Bagarazzi M L, Chattergoon M, Frost P, Javadian A, Williams W, Refaeli Y, Ciccarelli R B, McCallus D, Coney L, Weiner D B. Protection of chimpanzees from high dose heterologous HIV-1 challenge by DNA vaccination. Nat Med. 1997;3:526–532. - PubMed
-
- Crandell R A, Fabricant C G, Nelson-Rees W A. Development, characterisation and virus susceptibility of a feline (Felis catus) renal cell line (CRFK) In Vitro. 1973;9:176–185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

