Temporal analyses of virus replication, immune responses, and efficacy in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine
- PMID: 9696847
- PMCID: PMC109989
- DOI: 10.1128/JVI.72.9.7501-7509.1998
Temporal analyses of virus replication, immune responses, and efficacy in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine
Abstract
Despite evidence that live, attenuated simian immunodeficiency virus (SIV) vaccines can elicit potent protection against pathogenic SIV infection, detailed information on the replication kinetics of attenuated SIV in vivo is lacking. In this study, we measured SIV RNA in the plasma of 16 adult rhesus macaques immunized with a live, attenuated strain of SIV (SIVmac239Deltanef). To evaluate the relationship between replication of the vaccine virus and the onset of protection, four animals per group were challenged with pathogenic SIVmac251 at either 5, 10, 15, or 25 weeks after immunization. SIVmac239Deltanef replicated efficiently in the immunized macaques in the first few weeks after inoculation. SIV RNA was detected in the plasma of all animals by day 7 after inoculation, and peak levels of viremia (10(5) to 10(7) RNA copies/ml) occurred by 7 to 12 days. Following challenge, SIVmac251 was detected in all of the four animals challenged at 5 weeks, in two of four challenged at 10 weeks, in none of four challenged at 15 weeks, and one of four challenged at 25 weeks. One animal immunized with SIVmac239Deltanef and challenged at 10 weeks had evidence of disease progression in the absence of detectable SIVmac251. Although complete protection was not achieved at 5 weeks, a transient reduction in viremia (approximately 100-fold) occurred in the immunized macaques early after challenge compared to the nonimmunized controls. Two weeks after challenge, SIV RNA was also reduced in the lymph nodes of all immunized macaques compared with control animals. Taken together, these results indicate that host responses capable of reducing the viral load in plasma and lymph nodes were induced as early as 5 weeks after immunization with SIVmac239Deltanef, while more potent protection developed between 10 and 15 weeks. In further experiments, we found that resistance to SIVmac251 infection did not correlate with the presence of antibodies to SIV gp130 and p27 antigens and was achieved in the absence of significant neutralizing activity against the primary SIVmac251 challenge stock.
Figures
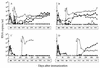
 )
was performed on day 0. The animals were then challenged with SIVmac251
(
)
was performed on day 0. The animals were then challenged with SIVmac251
( )
at either 5 (A), 10 (B), 15 (C), or 25 (D) weeks. Levels of SIV RNA for
the nonimmunized control animal in each challenge group are designated
by open symbols.
)
at either 5 (A), 10 (B), 15 (C), or 25 (D) weeks. Levels of SIV RNA for
the nonimmunized control animal in each challenge group are designated
by open symbols.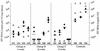

 )
and challenge with SIVmac251
(
)
and challenge with SIVmac251
( )
of animals in 5-week (A), 10-week (B), 15-week (C), and 25-week (D)
challenge groups. Data for nonimmunized control animals (1480, 1492,
1504, and 1516) are shown on the far right.
)
of animals in 5-week (A), 10-week (B), 15-week (C), and 25-week (D)
challenge groups. Data for nonimmunized control animals (1480, 1492,
1504, and 1516) are shown on the far right.
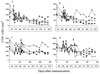
 ,
day of challenge with SIVmac251.
,
day of challenge with SIVmac251.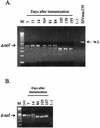
References
-
- Almond N, Cocoran T, Hull R, Walker B, Rose J, Sangster R, Silvera K, Silvera P, Cranage M, Rud E, Stott E J. Mechanisms of protection induced by attenuated simian immunodeficiency virus. I. Protection cannot be transferred with immune serum. J Gen Virol. 1997;78:1919–1922. - PubMed
-
- Almond N, Kent K, Cranage M, Rud E, Clarke B, Stott E J. Protection by attenuated simian immunodeficiency virus in macaques against challenge with virus-infected cells. Lancet. 1995;345:1342–1344. - PubMed
-
- Baba T W, Jeong Y S, Penninck D, Bronson R, Greene M F, Ruprecht R M. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267:1820–1825. - PubMed
-
- Burton, D. R., and D. Montefiori. 1997. The antibody response in HIV-1 infection. AIDS 11(Suppl. A):587–598. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

