The poliovirus empty capsid specifically recognizes the poliovirus receptor and undergoes some, but not all, of the transitions associated with cell entry
- PMID: 9696852
- PMCID: PMC109999
- DOI: 10.1128/JVI.72.9.7551-7556.1998
The poliovirus empty capsid specifically recognizes the poliovirus receptor and undergoes some, but not all, of the transitions associated with cell entry
Abstract
Experimental results presented here demonstrate that the poliovirus empty capsid binds with saturable character to poliovirus-susceptible cells, binds preferentially to susceptible cells, and competes with mature virus for binding sites on cells. Hence, induced changes in the structure and/or stability of the particle by RNA encapsidation and virus maturation are not necessary for recognition by receptor. In mature virus, heat-induced rearrangements mimic those induced by receptor at physiological temperatures in several important respects, namely, expulsion of VP4 and externalization of the VP1 N-terminal arm. It is shown here that in the empty capsid the VP1 N-terminal arm is externalized but the VP4 portion of VP0 is not. Thus, these two hallmark rearrangements associated with cell entry can be uncoupled.
Figures
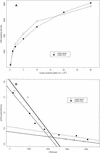
 , empty capsids;
▵, heated empty capsids; ◊, pentamers;
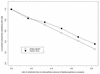
, empty capsids;
▵, heated empty capsids; ◊, pentamers;  , mature virus; ⧫,
135S; ▴, 80S. The dashed line in panel D represents the background
level for pentamer immunoprecipitation determined as described in the
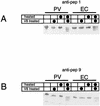
text. The reactivity of the undiluted anti-pep1 and anti-pep0
polyclonal antibodies toward unheated empty capsids may be due to
instability of the empty capsids in serum concentration. The high
background in control experiments using pentamers is due to
adventitious binding of pentamers to the protein A-Sepharose beads.
Mock experiments in which no anti-VP4 antibody was added resulted in
precipitation of the pentamers approximately equal to the background
level observed in panel D.
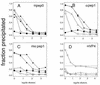
, mature virus; ⧫,
135S; ▴, 80S. The dashed line in panel D represents the background
level for pentamer immunoprecipitation determined as described in the
text. The reactivity of the undiluted anti-pep1 and anti-pep0
polyclonal antibodies toward unheated empty capsids may be due to
instability of the empty capsids in serum concentration. The high
background in control experiments using pentamers is due to
adventitious binding of pentamers to the protein A-Sepharose beads.
Mock experiments in which no anti-VP4 antibody was added resulted in
precipitation of the pentamers approximately equal to the background
level observed in panel D.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

