Detection of a trimeric human immunodeficiency virus type 1 Gag intermediate is dependent on sequences in the matrix protein, p17
- PMID: 9696871
- PMCID: PMC110034
- DOI: 10.1128/JVI.72.9.7659-7663.1998
Detection of a trimeric human immunodeficiency virus type 1 Gag intermediate is dependent on sequences in the matrix protein, p17
Abstract
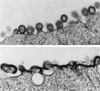
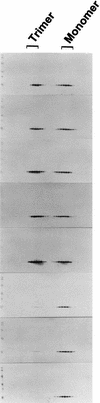
Previous studies have shown that single amino acid changes in the amino-terminal matrix (MA) domain, p17, of the human immunodeficiency virus type 1 Gag precursor Pr55, can abrogate virion particle assembly. In the three-dimensional structure of MA such mutations lie in a single helix spanning residues 54 to 68, suggesting a key role for this helix in the assembly process. The fundamental nature of this involvement, however, remains poorly understood. In the present study, the essential features of the MA helix required for virus assembly have been investigated through the analysis of a further 15 site-directed mutants. With previous mutants that failed to assemble, residues mapped as critical for assembly were all located on the hydrophobic face of the helix and had a key role in stabilizing the trimeric interface. This implies a role for the MA trimer in virus assembly. We support this interpretation by showing that purified MA is trimeric in solution and that mutations that prevent virus assembly also prevent trimerization. Trimerization in solution was also a property of a larger MA-capsid (CA) Gag molecule, while under the same conditions CA only was a monomer. These data suggest that Gag trimerization driven by the MA domain is an intermediate stage in normal virion assembly and that it relies, in turn, on an MA conformation dependent on the hydrophobic core of the molecule.
Figures

Similar articles
-
HIV-1 Matrix Trimerization-Impaired Mutants Are Rescued by Matrix Substitutions That Enhance Envelope Glycoprotein Incorporation.J Virol. 2019 Dec 12;94(1):e01526-19. doi: 10.1128/JVI.01526-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31619553 Free PMC article.
-
Human immunodeficiency virus type 1 MA deletion mutants expressed in baculovirus-infected cells: cis and trans effects on the Gag precursor assembly pathway.J Virol. 1995 Jan;69(1):365-75. doi: 10.1128/JVI.69.1.365-375.1995. J Virol. 1995. PMID: 7983731 Free PMC article.
-
Binding of human immunodeficiency virus type 1 Gag to membrane: role of the matrix amino terminus.J Virol. 1999 May;73(5):4136-44. doi: 10.1128/JVI.73.5.4136-4144.1999. J Virol. 1999. PMID: 10196310 Free PMC article.
-
Role of the Gag matrix domain in targeting human immunodeficiency virus type 1 assembly.J Virol. 2000 Mar;74(6):2855-66. doi: 10.1128/jvi.74.6.2855-2866.2000. J Virol. 2000. PMID: 10684302 Free PMC article.
-
Defined amino acids in the gag proteins of human immunodeficiency virus type 1 are functionally active during virus assembly.Intervirology. 1996;39(1-2):32-9. doi: 10.1159/000150472. Intervirology. 1996. PMID: 8957667 Review.
Cited by
-
Trimer Enhancement Mutation Effects on HIV-1 Matrix Protein Binding Activities.J Virol. 2016 May 27;90(12):5657-5664. doi: 10.1128/JVI.00509-16. Print 2016 Jun 15. J Virol. 2016. PMID: 27030269 Free PMC article.
-
Role of the HIV-1 Matrix Protein in Gag Intracellular Trafficking and Targeting to the Plasma Membrane for Virus Assembly.Front Microbiol. 2012 Feb 17;3:55. doi: 10.3389/fmicb.2012.00055. eCollection 2012. Front Microbiol. 2012. PMID: 22363329 Free PMC article.
-
An integrative bioinformatic approach for studying escape mutations in human immunodeficiency virus type 1 gag in the Pumwani Sex Worker Cohort.J Virol. 2008 Feb;82(4):1980-92. doi: 10.1128/JVI.02742-06. Epub 2007 Dec 5. J Virol. 2008. PMID: 18057233 Free PMC article.
-
Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor.Nat Med. 2008 Dec;14(12):1390-5. doi: 10.1038/nm.1779. Epub 2008 Nov 9. Nat Med. 2008. PMID: 18997777 Free PMC article.
-
Interaction Interface of Mason-Pfizer Monkey Virus Matrix and Envelope Proteins.J Virol. 2020 Sep 29;94(20):e01146-20. doi: 10.1128/JVI.01146-20. Print 2020 Sep 29. J Virol. 2020. PMID: 32796061 Free PMC article.
References
-
- Boulanger P, Jones I M. Use of heterologous expression systems to study retroviral morphogenesis. Curr Top Microbiol Immunol. 1996;214:237–260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

