alpha-Latrotoxin alters spontaneous and depolarization-evoked quantal release from rat adrenal chromaffin cells: evidence for multiple modes of action
- PMID: 9698306
- PMCID: PMC6793203
- DOI: 10.1523/JNEUROSCI.18-16-06113.1998
alpha-Latrotoxin alters spontaneous and depolarization-evoked quantal release from rat adrenal chromaffin cells: evidence for multiple modes of action
Abstract
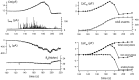
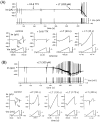
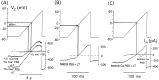
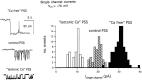
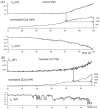
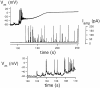
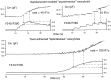
alpha-Latrotoxin (alpha-LT) potently enhances both "spontaneous" and "depolarization-evoked" quantal secretion from neurons. Here we have used the patch-clamped rat adrenal chromaffin cell to examine simultaneously the effects of alpha-LT on membrane current or voltage, cytosolic Ca, and membrane capacitance, the latter used as an assay for exocytosis. In chromaffin cells exposed to toxin concentrations of >100 pM, the development of large conductance, Ca-permeable ion channels, accompanied by a rise in cytosolic Ca to levels near 1 microM, precedes the initiation of spontaneous exocytosis. These channels appear to be induced de novo, because they occur concurrently with massive reduction or pharmacological block of voltage-dependent Na and Ca currents. However, enhancement of depolarization-evoked release, seen in many cells at <50 pM toxin, often occurs in the absence of a rise in background cytosolic Ca or de novo channel activity. These results favor Ca entry through toxin-induced channels underlying initiation of spontaneous release and direct modulation of the secretory machinery by the toxin-bound receptor contributing to enhancement of depolarization-evoked secretion as well as spontaneous release.
Figures


Similar articles
-
alpha-Latrotoxin increases spontaneous and depolarization-evoked exocytosis from pancreatic islet beta-cells.J Physiol. 2005 Jun 15;565(Pt 3):783-99. doi: 10.1113/jphysiol.2005.082586. Epub 2005 Mar 10. J Physiol. 2005. PMID: 15760942 Free PMC article.
-
alpha-Latrotoxin-induced quantal release of catecholamines from rat adrenal chromaffin cells.Brain Res. 1998 Jul 13;799(1):55-63. doi: 10.1016/s0006-8993(98)00467-3. Brain Res. 1998. PMID: 9666075
-
Ca(2+)-dependent K(+) current and exocytosis in responses to caffeine and muscarine in voltage-clamped guinea-pig adrenal chromaffin cells.J Neurochem. 2001 Sep;78(6):1243-55. doi: 10.1046/j.1471-4159.2001.00502.x. J Neurochem. 2001. PMID: 11579133
-
Calcium signaling and exocytosis in adrenal chromaffin cells.Physiol Rev. 2006 Oct;86(4):1093-131. doi: 10.1152/physrev.00039.2005. Physiol Rev. 2006. PMID: 17015485 Review.
-
Alpha-latrotoxin and its receptors CIRL (latrophilin) and neurexin 1 alpha mediate effects on secretion through multiple mechanisms.Biochimie. 2000 May;82(5):447-52. doi: 10.1016/s0300-9084(00)00222-4. Biochimie. 2000. PMID: 10865131 Review.
Cited by
-
Ca2+-secretion coupling is impaired in diabetic Goto Kakizaki rats.J Gen Physiol. 2007 Jun;129(6):493-508. doi: 10.1085/jgp.200609604. J Gen Physiol. 2007. PMID: 17535961 Free PMC article.
-
alpha-Latrotoxin and its receptors.Handb Exp Pharmacol. 2008;(184):171-206. doi: 10.1007/978-3-540-74805-2_7. Handb Exp Pharmacol. 2008. PMID: 18064415 Free PMC article. Review.
-
Catecholamine exocytosis during low frequency stimulation in mouse adrenal chromaffin cells is primarily asynchronous and controlled by the novel mechanism of Ca2+ syntilla suppression.J Physiol. 2014 Nov 1;592(21):4639-55. doi: 10.1113/jphysiol.2014.278127. Epub 2014 Aug 15. J Physiol. 2014. PMID: 25128575 Free PMC article.
-
alpha-Latrotoxin increases spontaneous and depolarization-evoked exocytosis from pancreatic islet beta-cells.J Physiol. 2005 Jun 15;565(Pt 3):783-99. doi: 10.1113/jphysiol.2005.082586. Epub 2005 Mar 10. J Physiol. 2005. PMID: 15760942 Free PMC article.
-
Structural basis of α-latrotoxin transition to a cation-selective pore.Nat Commun. 2024 Oct 3;15(1):8551. doi: 10.1038/s41467-024-52635-5. Nat Commun. 2024. PMID: 39362850 Free PMC article.
References
-
- Barnett DW, Liu J, Misler S. Single-cell measurements of quantal secretion induced by rat adrenal chromaffin cells: dependence on extracellular Ca2+. Pflügers Arch. 1996;432:1039–1046. - PubMed
-
- Barnett DW, Liu J, Misler S. Depolarization-induced asynchronous release from rat adrenal chromaffin cells. Biophys J. 1997;72:A157.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
