Calcium dependence and recovery kinetics of presynaptic depression at the climbing fiber to Purkinje cell synapse
- PMID: 9698309
- PMCID: PMC6793194
- DOI: 10.1523/JNEUROSCI.18-16-06147.1998
Calcium dependence and recovery kinetics of presynaptic depression at the climbing fiber to Purkinje cell synapse
Abstract
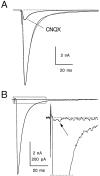
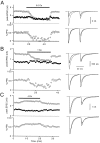
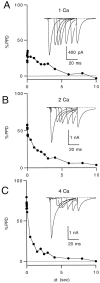
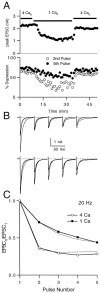
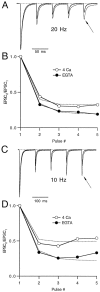
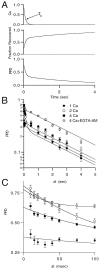
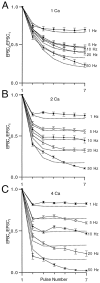
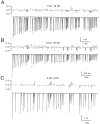
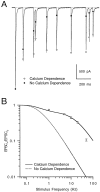
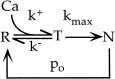
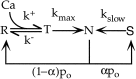
Short-term depression is a widespread form of use-dependent plasticity found in the peripheral and central nervous systems of invertebrates and vertebrates. The mechanism behind this transient decrease in synaptic strength is thought to be primarily the result of presynaptic "depletion" of a readily releasable neurotransmitter pool, which typically recovers with a time constant of a few seconds. We studied the mechanism and dynamics of recovery from depression at the climbing fiber to Purkinje cell synapse, where marked presynaptic depression has been described previously. Climbing fibers are well suited to studies of recovery from depression because they display little, if any, facilitation (even under conditions of low-release probability), which can obscure rapid recovery from depression for hundreds of milliseconds after release. We found that recovery from depression occurred in three kinetic phases. The fast and intermediate components could be approximated by exponentials with time constants of 100 msec and 3 sec at 24 degrees C. A much slower recovery phase was also present, but it was only prominent during prolonged stimulus trains. The fast component was enhanced by raising extracellular calcium and was eliminated by lowering presynaptic calcium, suggesting that, on short time scales, recovery from depression is driven by residual calcium. During regular and Poisson stimulus trains, recovery from depression was dramatically accelerated by accumulation of presynaptic residual calcium, maintaining synaptic efficacy under conditions that would otherwise deplete the available transmitter pool. This represents a novel form of presynaptic plasticity in that high levels of activity modulate the rate of recovery as well as the magnitude of depression.
Figures




References
-
- Abbott LF, Sen K, Varela JA, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–222. - PubMed
-
- Artalejo CR, Elhamdani A, Palfrey HC. Calmodulin is the divalent cation receptor for rapid endocytosis, but not exocytosis, in adrenal chromaffin cells. Neuron. 1996;16:195–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
