Tenascin-R is antiadhesive for activated microglia that induce downregulation of the protein after peripheral nerve injury: a new role in neuronal protection
- PMID: 9698315
- PMCID: PMC6793216
- DOI: 10.1523/JNEUROSCI.18-16-06218.1998
Tenascin-R is antiadhesive for activated microglia that induce downregulation of the protein after peripheral nerve injury: a new role in neuronal protection
Abstract
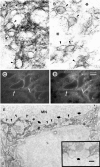
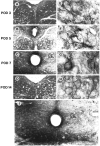
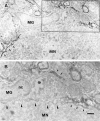
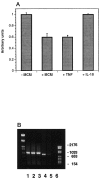
Microglial activation in response to pathological stimuli is characterized by increased migratory activity and potential cytotoxic action on injured neurons during later stages of neurodegeneration. The initial molecular changes in the CNS favoring neuronofugal migration of microglia remain, however, largely unknown. We report that the extracellular matrix protein tenascin-R (TN-R) present in the intact CNS is antiadhesive for activated microglia, and its downregulation after facial nerve axotomy may account for the loss of motoneuron protection and subsequent neurodegeneration. Studies on the protein expression in the facial and hypoglossal nucleus in rats demonstrate that TN-R is a constituent of the perineuronal net of motoneurons and 7 d after peripheral nerve injury becomes downregulated in the corresponding motor nucleus. This downregulation is reversible under regenerative (nerve suture) conditions and irreversible under degenerative (nerve resection) conditions. In short-term adhesion assays, the unlesioned side of brainstem cryosections from unilaterally operated animals is nonpermissive for activated microglia, and this nonpermissiveness is almost abolished by a monoclonal antibody to TN-R. Microglia-conditioned media and tumor necrosis factor-alpha downregulate TN-R protein and mRNA synthesis by cultured oligodendrocytes, which are one of the sources for TN-R in the brainstem. Our findings suggest a new role for TN-R in neuronal protection against activated microglia and the participation of the latter in perineuronal net destruction, e.g., downregulation of TN-R.
Figures



References
-
- Aldskogius H, Thomander L. Selective reinnervation of somatotopically appropriate muscles after facial nerve transection and regeneration in the neonatal rat. Brain Res. 1986;375:126–134. - PubMed
-
- Angelov DN, Neiss WF. Phagocytic microglia in the intracerebral presentation of antigen to the immune system. Morphological correlates. In: Ling EA, Tan CK, Tan CBC, editors. Topical issues in microglia research. Goh Brothers Enterprise Humanities; Singapore: 1996. pp. 165–188.
-
- Angelov DN, Gunkel A, Stennert E, Neiss WF. Phagocytic microglia during delayed neuronal loss in the facial nucleus of the rat: time course of the neuronofugal migration of brain macrophages. Glia. 1995;13:113–129. - PubMed
-
- Angelov DN, Neiss WF, Streppel M, Walther M, Guntinas-Lichius O, Stennert E. ED2-positive perivascular cells act as neuronophages during delayed neuronal loss in the facial nucleus of the rat. Glia. 1996b;16:129–139. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
