Localization of Ca2+ channel subtypes on rat spinal motor neurons, interneurons, and nerve terminals
- PMID: 9698323
- PMCID: PMC6793183
- DOI: 10.1523/JNEUROSCI.18-16-06319.1998
Localization of Ca2+ channel subtypes on rat spinal motor neurons, interneurons, and nerve terminals
Abstract
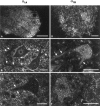
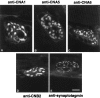
Ca2+ channels in distinct subcellular compartments of neurons mediate voltage-dependent Ca2+ influx, which integrates synaptic responses, regulates gene expression, and initiates synaptic transmission. Antibodies that specifically recognize the alpha1 subunits of class A, B, C, D, and E Ca2+ channels have been used to investigate the localization of these voltage-gated ion channels on spinal motor neurons, interneurons, and nerve terminals of the adult rat. Class A P/Q-type Ca2+ channels were present mainly in a punctate pattern in nerve terminals located along the cell bodies and dendrites of motor neurons. Both smooth and punctate staining patterns were observed over the surface of the cell bodies and dendrites with antibodies to class B N-type Ca2+ channels, indicating the presence of these channels in the cell surface membrane and in nerve terminals. Class C and D L-type and class E R-type Ca2+ channels were distributed mainly over the cell soma and proximal dendrites. Class A P/Q-type Ca2+ channels were present predominantly in the presynaptic terminals of motor neurons at the neuromuscular junction. Occasional nerve terminals innervating skeletal muscles from the hindlimb were labeled with antibodies against class B N-type Ca2+ channels. Staining of the dorsal laminae of the rat spinal cord revealed a complementary distribution of class A and class B Ca2+ channels in nerve terminals in the deeper versus the superficial laminae. Many of the nerve terminals immunoreactive for class B N-type Ca2+ channels also contained substance P, an important neuropeptide in pain pathways, suggesting that N-type Ca2+ channels are predominant at synapses that carry nociceptive information into the spinal cord.
Figures





References
-
- Appel SH, Stefani E. Amyotrophic lateral sclerosis: etiology and pathogenesis. In: Appel SH, editor. Current neurology, Vol 11. Mosby; Chicago: 1991. pp. 287–310.
-
- Appel SH, Smith RG, Engelhardt JI, Stefani E. Evidence for autoimmunity in amyotrophic lateral sclerosis. J Neurol Sci. 1993;118:169–174. - PubMed
-
- Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct Ca2+ signaling pathways. Science. 1993;260:181–186. - PubMed
-
- Basbaum AI, Fields HL. Endogenous pain control system: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
