Computational models of thalamocortical augmenting responses
- PMID: 9698334
- PMCID: PMC6793176
- DOI: 10.1523/JNEUROSCI.18-16-06444.1998
Computational models of thalamocortical augmenting responses
Abstract
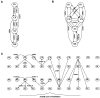
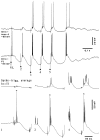
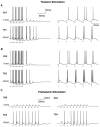
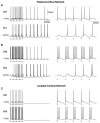
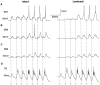
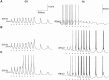
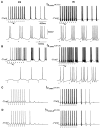
Repetitive stimulation of the dorsal thalamus at 7-14 Hz produces an increasing number of spikes at an increasing frequency in neocortical neurons during the first few stimuli. Possible mechanisms underlying these cortical augmenting responses were analyzed with a computer model that included populations of thalamocortical cells, thalamic reticular neurons, up to two layers of cortical pyramidal cells, and cortical inhibitory interneurons. Repetitive thalamic stimulation produced a low-threshold intrathalamic augmentation in the model based on the deinactivation of the low-threshold Ca2+ current in thalamocortical cells, which in turn induced cortical augmenting responses. In the cortical model, augmenting responses were more powerful in the "input" layer compared with those in the "output" layer. Cortical stimulation of the network model produced augmenting responses in cortical neurons in distant cortical areas through corticothalamocortical loops and low-threshold intrathalamic augmentation. Thalamic stimulation was more effective in eliciting augmenting responses than cortical stimulation. Intracortical inhibition had an important influence on the genesis of augmenting responses in cortical neurons: A shift in the balance between intracortical excitation and inhibition toward excitation transformed an augmenting responses to long-lasting paroxysmal discharge. The predictions of the model were compared with in vivo recordings from neurons in cortical area 4 and thalamic ventrolateral nucleus of anesthetized cats. The known intrinsic properties of thalamic cells and thalamocortical interconnections can account for the basic properties of cortical augmenting responses.
Figures








References
-
- Avoli M. Inhibitory potentials in neurons of the deep layers of the in vitro neocortical slices. Brain Res. 1986;370:165–170. - PubMed
-
- Bal T, McCormick DA. What stops synchronized thalamocortical oscillations? Neuron. 1996;17:297–308. - PubMed
-
- Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Cellular and network models for intrathalamic augmenting responses during 10 Hz stimulation. J Neurophysiol. 1998;79:2730–2748. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
