Neurofilament proteins in Y-cells of the cat lateral geniculate nucleus: normal expression and alteration with visual deprivation
- PMID: 9698342
- PMCID: PMC6793172
- DOI: 10.1523/JNEUROSCI.18-16-06549.1998
Neurofilament proteins in Y-cells of the cat lateral geniculate nucleus: normal expression and alteration with visual deprivation
Abstract
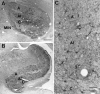
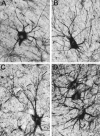
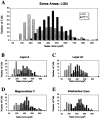
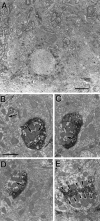
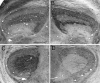
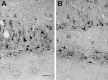
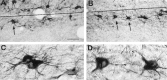
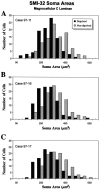
We examined neurofilament staining in the normal and visually deprived lateral geniculate nucleus (LGN), using the SMI-32 antibody. This antibody preferentially stains LGN cells that display the morphological characteristics of Y-cells. The soma sizes of SMI-32-stained cells were consistent with those of the overall population of Y-cells, and the Golgi-like staining of their dendrites revealed a radial distribution that often crossed laminar boundaries. Labeled cells were distributed within the A laminae (primarily near laminar borders), the magnocellular portion of the C laminae, and the medial intralaminar nucleus, but they were absent in the parvocellular C laminae. Electron microscopic examination of SMI-32-stained tissue revealed that staining was confined to somata, dendrites, and large myelinated axons. Retinal synapses on SMI-32-labeled dendrites were primarily simple axodendritic contacts; few triadic arrangements were observed. In the LGN of cats reared with monocular lid suture, SMI-32 staining was decreased significantly in the A laminae that received input from the deprived eye. Dephosphorylation of the tissue did not alter the cellular SMI-32 staining patterns. Analysis of staining patterns in the C laminae and monocular zone of the A laminae suggests that changes in the cytoskeleton after lid suture reflect cell class and not binocular competition. Taken together, the results from normal and lid-sutured animals suggest that the cat LGN offers a unique model system in which the cytoskeleton of one class of cells can be manipulated by altering neuronal activity.
Figures


References
-
- Adams JC. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981;29:775. - PubMed
-
- Bickford ME, Van Horn SC, Sherman SM. Retinal Y axons contact interneurons in the lateral geniculate nucleus of the cat. Soc Neurosci Abstr. 1992;18:141.
-
- Campbell MJ, Morrison JH. Monoclonal antibody to neurofilament protein (SMI-32) labels a subpopulation of pyramidal neurons in the human and monkey cortex. J Comp Neurol. 1989;282:191–205. - PubMed
-
- Carmichael ST, Price JL. Architectonic subdivision of the orbital and prefrontal cortex in the macaque monkey. J Comp Neurol. 1994;346:366–402. - PubMed
-
- Chaudhuri A, Zangenehpour S, Matsubara JA, Cynader MS. Differential expression of neurofilament protein in the visual system of the vervet monkey. Brain Res. 1996;709:17–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
