Involvement of hydrogen peroxide in the differentiation of clonal HD-11EM cells into osteoclast-like cells
- PMID: 9699510
- PMCID: PMC2950278
- DOI: 10.1002/(SICI)1097-4652(199809)176:3<574::AID-JCP14>3.0.CO;2-#
Involvement of hydrogen peroxide in the differentiation of clonal HD-11EM cells into osteoclast-like cells
Abstract
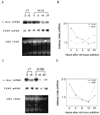
The present study uses the osteoclast precursor clonal line, HD-11EM, to study the potential of hydrogen peroxide (H2O2) in mediating the differentiation of HD-11EM into osteoclast-like cells. HD-11EM cells are a newly established clonal cell line that, in response to 1alpha,25-(OH)2D3, differentiate into osteoclast-like cells that are multinucleated (more than three nuclei), express tartrate-resistant acid phosphatase (TRAP), and excavate resorption pits when cultured on dentin slices in the presence of osteoblasts (Hsia et al., 1995, J. Bone Miner. Res., 10(Suppl 1):S424; Hsia, and Hauschka, 1997, unpublished data). Here we demonstrate that HD-11EM express the reduced nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase specific cytochrome b558 subunits, and that stimulation of HD-11EM with 1 or 10 nM 1alpha,25-(OH)2D3 increases the extracellular release of H2O2 within 5-10 min. Ours is the first report that stimulation of a cell with 1alpha,25-(OH)2D3 enhances the activation of NADPH-oxidase and increases the basal release of superoxide and the formation of its dismutation product, H2O2. To determine the possible involvement of H2O2 in the differentiation of HD-11EM, these cells were exposed to glucose/glucose oxidase. This enzyme system was used to deliver a pure and continuous source of H2O2 in nanomole amounts consistent with quantities produced by HD-11EM in response to 1alpha,25-(OH)2D3. Both 1alpha,25-(OH)2D3 and the exogenously generated H2O2 stimulated a dose- and time-dependent increase in TRAP activity/cell and the number of multinucleated cells 24-48 hr after treatment. Northern analysis confirmed an increase in expression of TRAP mRNA in response to either 1alpha,25-(OH)2D3 or H2O2. Decreases in cell proliferation and v-myc mRNA were also observed in response to these agents. Taken together, our findings indicate that production of H2O2 by HD-11EM is an important local factor involved in differentiation of HD-11EM into osteoclast-like cells, and suggest that H2O2 may play a role in native osteoclast differentiation.
Figures





Similar articles
-
Regulation of matrix metalloproteinase-9 protein expression by 1α, 25-(OH)₂D₃ during osteoclast differentiation.J Vet Sci. 2014;15(1):133-40. doi: 10.4142/jvs.2014.15.1.133. Epub 2013 Oct 18. J Vet Sci. 2014. PMID: 24136216 Free PMC article.
-
Transcription from the tartrate-resistant acid phosphatase promoter is negatively regulated by the Myc oncoprotein.J Bone Miner Res. 2002 Sep;17(9):1701-9. doi: 10.1359/jbmr.2002.17.9.1701. J Bone Miner Res. 2002. PMID: 12211441
-
17Beta-estradiol antagonizes effects of 1alpha,25-dihydroxyvitamin D3 on interleukin-6 production and osteoclast-like cell formation in mouse bone marrow primary cultures.Endocrinology. 1997 Nov;138(11):4567-71. doi: 10.1210/endo.138.11.5523. Endocrinology. 1997. PMID: 9348179
-
Interaction of triiodothyronine with 1alpha,25-dihydroxyvitamin D3 on interleukin-6-dependent osteoclast-like cell formation in mouse bone marrow cell cultures.Bone. 1998 Apr;22(4):341-6. doi: 10.1016/s8756-3282(97)00291-3. Bone. 1998. PMID: 9556133
-
Osteoclast differentiation in cocultures of a clonal chondrogenic cell line and mouse bone marrow cells.Endocrinology. 1993 Nov;133(5):2292-300. doi: 10.1210/endo.133.5.7691585. Endocrinology. 1993. PMID: 7691585
Cited by
-
Skeletal cell differentiation is enhanced by atmospheric dielectric barrier discharge plasma treatment.PLoS One. 2013 Dec 12;8(12):e82143. doi: 10.1371/journal.pone.0082143. eCollection 2013. PLoS One. 2013. PMID: 24349203 Free PMC article.
-
Endogenous hydrogen peroxide production in the epithelium of the developing embryonic lens.Mol Vis. 2014 Apr 11;20:458-67. eCollection 2014. Mol Vis. 2014. PMID: 24744606 Free PMC article.
-
Rosmarinic acid and arbutin suppress osteoclast differentiation by inhibiting superoxide and NFATc1 downregulation in RAW 264.7 cells.Biomed Rep. 2015 Jul;3(4):483-490. doi: 10.3892/br.2015.452. Epub 2015 Apr 17. Biomed Rep. 2015. PMID: 26171153 Free PMC article.
-
DJ-1 controls bone homeostasis through the regulation of osteoclast differentiation.Nat Commun. 2017 Nov 15;8(1):1519. doi: 10.1038/s41467-017-01527-y. Nat Commun. 2017. PMID: 29142196 Free PMC article.
-
Role of NADPH oxidase in formation and function of multinucleated giant cells.J Innate Immun. 2009;1(6):509-26. doi: 10.1159/000228158. Epub 2009 Jul 7. J Innate Immun. 2009. PMID: 20375608 Free PMC article. Review.
References
-
- Athanasou NA. Current concepts review cellular biology of bone-resorbing cells. J. Bone Joint Surg. Am. 1996;78:1096–1112. - PubMed
-
- Bax BE, Alam ASMT, Banerji B, Bax CMR, Bevis PJR, Stevens CR, Moonga BS, Blake DR, Zaidi M. Stimulation of osteoclastic bone resorption by hydrogen peroxide. Biochem. Biophys. Res. Commun. 1992;183:1153–1158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

