The sorting of mitochondrial DNA and mitochondrial proteins in zygotes: preferential transmission of mitochondrial DNA to the medial bud
- PMID: 9700153
- PMCID: PMC2148178
- DOI: 10.1083/jcb.142.3.613
The sorting of mitochondrial DNA and mitochondrial proteins in zygotes: preferential transmission of mitochondrial DNA to the medial bud
Abstract
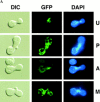
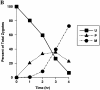
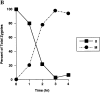
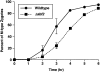
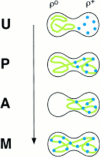
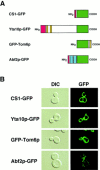
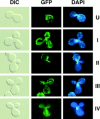
Green fluorescent protein (GFP) was used to tag proteins of the mitochondrial matrix, inner, and outer membranes to examine their sorting patterns relative to mtDNA in zygotes of synchronously mated yeast cells in rho+ x rho0 crosses. When transiently expressed in one of the haploid parents, each of the marker proteins distributes throughout the fused mitochondrial reticulum of the zygote before equilibration of mtDNA, although the membrane markers equilibrate slower than the matrix marker. A GFP-tagged form of Abf2p, a mtDNA binding protein required for faithful transmission of rho+ mtDNA in vegetatively growing cells, colocalizes with mtDNA in situ. In zygotes of a rho+ x rho+ cross, in which there is little mixing of parental mtDNAs, Abf2p-GFP prelabeled in one parent rapidly equilibrates to most or all of the mtDNA, showing that the mtDNA compartment is accessible to exchange of proteins. In rho+ x rho0 crosses, mtDNA is preferentially transmitted to the medial diploid bud, whereas mitochondrial GFP marker proteins distribute throughout the zygote and the bud. In zygotes lacking Abf2p, mtDNA sorting is delayed and preferential sorting is reduced. These findings argue for the existence of a segregation apparatus that directs mtDNA to the emerging bud.
Figures






References
-
- Arlt H, Tauer R, Feldmann H, Neupert W, Langer T. The YTA10-12 complex, an AAA protease with chaperone-like activity in the inner membrane of mitochondria. Cell. 1996;85:875–885. - PubMed
-
- Azpiroz R, Butow RA. Mitochondrial inheritance in yeast. Methods Enzymol. 1995;260:453–466. - PubMed
-
- Berger KH, Yaffe MP. Mitochondrial distribution and inheritance. Experentia. 1996;52:1111–1116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

