Dual roles for Ste24p in yeast a-factor maturation: NH2-terminal proteolysis and COOH-terminal CAAX processing
- PMID: 9700155
- PMCID: PMC2148179
- DOI: 10.1083/jcb.142.3.635
Dual roles for Ste24p in yeast a-factor maturation: NH2-terminal proteolysis and COOH-terminal CAAX processing
Abstract
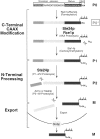
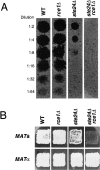
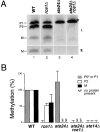
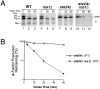
Maturation of the Saccharomyces cerevisiae a-factor precursor involves COOH-terminal CAAX processing (prenylation, AAX tripeptide proteolysis, and carboxyl methylation) followed by cleavage of an NH2-terminal extension (two sequential proteolytic processing steps). The aim of this study is to clarify the precise role of Ste24p, a membrane-spanning zinc metalloprotease, in the proteolytic processing of the a-factor precursor. We demonstrated previously that Ste24p is necessary for the first NH2-terminal processing step by analysis of radiolabeled a-factor intermediates in vivo (Fujimura-Kamada, K., F.J. Nouvet, and S. Michaelis. 1997. J. Cell Biol. 136:271-285). In contrast, using an in vitro protease assay, others showed that Ste24p (Afc1p) and another gene product, Rce1p, share partial overlapping function as COOH-terminal CAAX proteases (Boyartchuk, V.L., M.N. Ashby, and J. Rine. 1997. Science. 275:1796-1800). Here we resolve these apparently conflicting results and provide compelling in vivo evidence that Ste24p indeed functions at two steps of a-factor maturation using two methods. First, direct analysis of a-factor biosynthetic intermediates in the double mutant (ste24Delta rce1Delta) reveals a previously undetected species (P0*) that fails to be COOH terminally processed, consistent with redundant roles for Ste24p and Rce1p in COOH-terminal CAAX processing. Whereas a-factor maturation appears relatively normal in the rce1Delta single mutant, the ste24Delta single mutant accumulates an intermediate that is correctly COOH terminally processed but is defective in cleavage of the NH2-terminal extension, demonstrating that Ste24p is also involved in NH2-terminal processing. Together, these data indicate dual roles for Ste24p and a single role for Rce1p in a-factor processing. Second, by using a novel set of ubiquitin-a-factor fusions to separate the NH2- and COOH-terminal processing events of a-factor maturation, we provide independent evidence for the dual roles of Ste24p. We also report here the isolation of the human (Hs) Ste24p homologue, representing the first human CAAX protease to be cloned. We show that Hs Ste24p complements the mating defect of the yeast double mutant (ste24Delta rce1Delta) strain, implying that like yeast Ste24p, Hs Ste24p can mediate multiple types of proteolytic events.
Figures



References
-
- Adames N, Blundell K, Ashby MN, Boone C. Role of yeast insulin-degrading enzyme homologs in propheromone processing and bud site selection. Science. 1995;270:464–467. - PubMed
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Andres DA, Milatovich A, Ozcelik T, Wenzlau JM, Brown MS, Goldstein JL, Francke U. cDNA cloning of the two subunits of human CAAX farnesyltransferase and chromosomal mapping of FNTA and FNTB loci and related sequences. Genomics. 1993;18:105–112. - PubMed
-
- Ashby MN, Rine J. Ras and a-factor converting enzyme. Methods Enzymol. 1995;250:235–250. - PubMed
-
- Ausubel, F.M., R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl. 1987. Current Protocols in Molecular Biology. Vol. 1. Greene Publishing Associates, New York.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

