Retrograde traffic out of the yeast vacuole to the TGN occurs via the prevacuolar/endosomal compartment
- PMID: 9700156
- PMCID: PMC2148167
- DOI: 10.1083/jcb.142.3.651
Retrograde traffic out of the yeast vacuole to the TGN occurs via the prevacuolar/endosomal compartment
Abstract
A large number of trafficking steps occur between the last compartment of the Golgi apparatus (TGN) and the vacuole of the yeast Saccharomyces cerevisiae. To date, two intracellular routes from the TGN to the vacuole have been identified. Carboxypeptidase Y (CPY) travels through a prevacuolar/endosomal compartment (PVC), and subsequently on to the vacuole, while alkaline phosphatase (ALP) bypasses this compartment to reach the same organelle. Proteins resident to the TGN achieve their localization despite a continuous flux of traffic by continually being retrieved from the distal PVC by virtue of an aromatic amino acid-containing sorting motif. In this study we report that a hybrid protein based on ALP and containing this retrieval motif reaches the PVC not by following the CPY sorting pathway, but instead by signal-dependent retrograde transport from the vacuole, an organelle previously thought of as a terminal compartment. In addition, we show that a mutation in VAC7, a gene previously identified as being required for vacuolar inheritance, blocks this trafficking step. Finally we show that Vti1p, a v-SNARE required for the delivery of both CPY and ALP to the vacuole, uses retrograde transport out of the vacuole as part of its normal cellular itinerary.
Figures


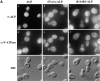






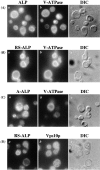
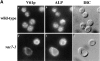


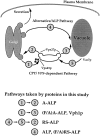
References
-
- Akasaki K, Fukuzawa M, Kinoshita H, Furuno K, Tsuji H. Cycling of two endogenous lysosomal membrane proteins, lamp-2 and acid phosphatase, between the cell surface and lysosomes in cultured rat hepatocytes. J Biochem (Tokyo) 1993;114:598–604. - PubMed
-
- Akasaki K, Michinhara A, Mibuka K, Fujiwara Y, Tsuji H. Biosynthetic transport of a major lysosomal membrane glycoprotein, lamp-1: convergence of biosynthetic and endocytic pathways occurs at three distinctive points. Exp Cell Res. 1995;220:464–473. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

