A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast
- PMID: 9700157
- PMCID: PMC2148169
- DOI: 10.1083/jcb.142.3.665
A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast
Abstract
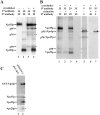
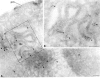
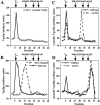
We have recently characterized three yeast gene products (Vps35p, Vps29p, and Vps30p) as candidate components of the sorting machinery required for the endosome-to-Golgi retrieval of the vacuolar protein sorting receptor Vps10p (Seaman, M.N.J., E.G. Marcusson, J.-L. Cereghino, and S.D. Emr. 1997. J. Cell Biol. 137:79-92). By genetic and biochemical means we now show that Vps35p and Vps29p interact and form part of a multimeric membrane-associated complex that also contains Vps26p, Vps17p, and Vps5p. This complex, designated here as the retromer complex, assembles from two distinct subcomplexes comprising (a) Vps35p, Vps29p, and Vps26p; and (b) Vps5p and Vps17p. Density gradient fractionation of Golgi/endosomal/vesicular membranes reveals that Vps35p cofractionates with Vps5p/Vps17p in a vesicle-enriched dense membrane fraction. Furthermore, gel filtration analysis indicates that Vps35p and Vps5p are present on a population of vesicles and tubules slightly larger than COPI/coatomer-coated vesicles. We also show by immunogold EM that Vps5p is localized to discrete regions at the rims of the prevacuolar endosome where vesicles appear to be budding. Size fractionation of cytosolic and recombinant Vps5p reveals that Vps5p can self-assemble in vitro, suggesting that Vps5p may provide the mechanical impetus to drive vesicle formation. Based on these findings we propose a model in which Vps35p/Vps29p/Vps26p function to select cargo for retrieval, and Vps5p/Vps17p assemble onto the membrane to promote vesicle formation. Conservation of the yeast retromer complex components in higher eukaryotes suggests an important general role for this complex in endosome-to-Golgi retrieval.
Figures

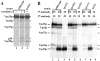






References
-
- Bachhawat AK, Suhan J, Jones EW. The yeast homologue of Hβ58, a mouse gene essential for embryogenesis performs a role in the delivery of proteins to the vacuole. Genes Dev. 1994;8:1379–1387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

