Spindle self-organization and cytokinesis during male meiosis in asterless mutants of Drosophila melanogaster
- PMID: 9700163
- PMCID: PMC2148166
- DOI: 10.1083/jcb.142.3.751
Spindle self-organization and cytokinesis during male meiosis in asterless mutants of Drosophila melanogaster
Abstract
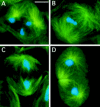
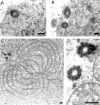
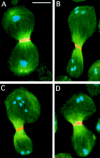
While Drosophila female meiosis is anastral, both meiotic divisions in Drosophila males exhibit prominent asters. We have identified a gene we call asterless (asl) that is required for aster formation during male meiosis. Ultrastructural analysis showed that asl mutants have morphologically normal centrioles. However, immunostaining with antibodies directed either to gamma tubulin or centrosomin revealed that these proteins do not accumulate in the centrosomes, as occurs in wild-type. Thus, asl appears to specify a function required for the assembly of centrosomal material around the centrioles. Despite the absence of asters, meiotic cells of asl mutants manage to develop an anastral spindle. Microtubules grow from multiple sites around the chromosomes, and then focus into a peculiar bipolar spindle that mediates chromosome segregation, although in a highly irregular way. Surprisingly, asl spermatocytes eventually form a morphologically normal ana-telophase central spindle that has full ability to stimulate cytokinesis. These findings challenge the classical view on central spindle assembly, arguing for a self-organization of this structure from either preexisting or newly formed microtubules. In addition, these findings strongly suggest that the asters are not required for signaling cytokinesis.
Figures




References
-
- Albertson DG, Thomson JN. Segregation of holocentric chromosomes at meiosis in the nematode Caenorhabditis elegans. . Chromosome Res. 1993;1:15–26. - PubMed
-
- Ault JG, Rieder CL. Centrosome and kinetochore movement during mitosis. Curr Opin Cell Biol. 1994;6:41–49. - PubMed
-
- Callaini G, Whitfield WG, Riparbelli MG. Centriole and centrosome dynamics during the embryonic cell cycles that follow the formation of the cellular blastoderm in Drosophila. . Exp Cell Res. 1997;234:183–190. - PubMed
-
- Cenci G, Bonaccorsi S, Pisano C, Verni F, Gatti M. Chromatin and microtubule organization during premeiotic, meiotic, and early postmeiotic stages of Drosophila melanogasterspermatogenesis. J Cell Sci. 1994;107:3521–3534. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

