Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes
- PMID: 9700169
- PMCID: PMC2148160
- DOI: 10.1083/jcb.142.3.827
Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes
Abstract
Melanocyte differentiation characterized by an increased melanogenesis, is stimulated by alpha-melanocyte-stimulating hormone through activation of the cAMP pathway. During this process, the expression of tyrosinase, the enzyme that controls melanin synthesis is upregulated. We previously showed that cAMP regulates transcription of the tyrosinase gene through a CATGTG motif that binds microphthalmia a transcription factor involved in melanocyte survival. Further, microphthalmia stimulates the transcriptional activity of the tyrosinase promoter and cAMP increases the binding of microphthalmia to the CATGTG motif. These observations led us to hypothesize that microphthalmia mediates the effect of cAMP on the expression of tyrosinase. The present study was designed to elucidate the mechanism by which cAMP regulates microphthalmia function and to prove our former hypothesis, suggesting that microphthalmia is a key component in cAMP-induced melanogenesis. First, we showed that cAMP upregulates the transcription of microphthalmia gene through a classical cAMP response element that is functional only in melanocytes. Then, using a dominant-negative mutant of microphthalmia, we demonstrated that microphthalmia is required for the cAMP effect on tyrosinase promoter. These findings disclose the mechanism by which cAMP stimulates tyrosinase expression and melanogenesis and emphasize the critical role of microphthalmia as signal transducer in cAMP-induced melanogenesis and pigment cell differentiation.
Figures




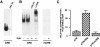

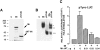
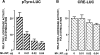
References
-
- Abdel-Malek A, Swope BV, Amornsiripanitch N, Nordlund JJ. In vitro modulation of proliferation and melanization of S91 melanoma cells by prostaglandins. Cancer Res. 1987;47:3141–3146. - PubMed
-
- Arany Z, Newsome D, Olread D, Livingston DM, Eckner R. A family of transcriptional adaptator proteins targeted by E1A oncoprotein. Nature. 1995;374:81–84. - PubMed
-
- Arias J, Alberts AS, Brindle P, Claret FX, Smeal T, Karim M, Ferasmisco J, Montminy M. Activation of cAMP and mitogen responsive genes relies on a commun nuclear factor. Nature. 1994;370:226–229. - PubMed
-
- Barber JI, Townsend D, Olds DP, King RA. Dopachrome oxydoreductase: a new enzyme in the pigment pathway. J Invest Dermatol. 1984;83:145–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

