Adhesive but not lateral E-cadherin complexes require calcium and catenins for their formation
- PMID: 9700170
- PMCID: PMC2148173
- DOI: 10.1083/jcb.142.3.837
Adhesive but not lateral E-cadherin complexes require calcium and catenins for their formation
Abstract
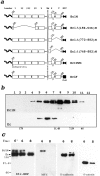
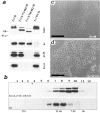
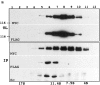
We examined intercadherin interactions in epithelial A-431 cells producing endogenous E-cadherin and recombinant forms of E-cadherin tagged either by myc or by flag epitopes. Three distinct E-cadherin complexes were found. The first is a conventional E-cadherin-catenin complex consisting of one E-cadherin molecule linked either to beta-catenin/alpha-catenin or to plakoglobin/alpha-catenin dimers. The second is a lateral E-cadherin complex incorporating two E-cadherin- catenin conventional complexes combined in parallel fashion via dimerization of the NH2-terminal extracellular domain of E-cadherin. The third complex is likely to contain two E-cadherin-catenin conventional complexes derived from two opposing cells and arranged in an antiparallel fashion. Formation of the antiparallel but not lateral complex strictly depends on extracellular calcium and E-cadherin binding to catenins. Double amino acid substitution Trp156Ala/Val157Gly within the extracellular NH2-terminal E-cadherin domain completely abolished both lateral and antiparallel inter-E-cadherin association. These data support an idea that the antiparallel complex has the adhesion function. Furthermore, they allow us to suggest that antiparallel complexes derive from lateral dimers and this complex process requires catenins and calcium ions.
Figures

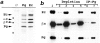


References
-
- Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198:11–26. - PubMed
-
- Blaschuk OW, Sullivan R, David S, Pouliot Y. Identification of a cadherin cell adhesion recognition sequence. Dev Biol. 1990;139:227–229. - PubMed
-
- Bussemakers MJ, van Bokhoven A, Mees SG, Kemler R, Schalken JA. Molecular cloning and characterization of the human E-cadherin cDNA. Mol Biol Rep. 1993;17:123–128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

