alpha-Catenin-vinculin interaction functions to organize the apical junctional complex in epithelial cells
- PMID: 9700171
- PMCID: PMC2148175
- DOI: 10.1083/jcb.142.3.847
alpha-Catenin-vinculin interaction functions to organize the apical junctional complex in epithelial cells
Abstract
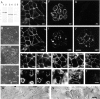
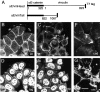
alphaE-catenin, a cadherin-associated protein, is required for tight junction (TJ) organization, but its role is poorly understood. We transfected an alphaE-catenin-deficient colon carcinoma line with a series of alphaE-catenin mutant constructs. The results showed that the amino acid 326-509 domain of this catenin was required to organize TJs, and its COOH-terminal domain was not essential for this process. The 326-509 internal domain was found to bind vinculin. When an NH2-terminal alphaE-catenin fragment, which is by itself unable to organize the TJ, was fused with the vinculin tail, this chimeric molecule could induce TJ assembly in the alphaE-catenin-deficient cells. In vinculin-null F9 cells, their apical junctional organization was impaired, and this phenotype was rescued by reexpression of vinculin. These results indicate that the alphaE-catenin-vinculin interaction plays a role in the assembly of the apical junctional complex in epithelia.
Figures






References
-
- Barth AIM, Näthke IS, Nelson WJ. Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol. 1997;9:683–690. - PubMed
-
- Belkin AM, Koteliansky VE. Interaction of iodinated vinculin, metavinculin and alpha-actinin with cytoskeletal proteins. FEBS Lett. 1987;220:291–294. - PubMed
-
- Bornslaeger EA, Corcoran CM, Stappenbeck TS, Green KJ. Breaking the connection: displacement of the desmosomal plaque protein desmoplakin from cell-cell interfaces disrupts anchorage of intermediate filament bundles and alters intercellular junction assembly. J Cell Biol. 1996;134:985–1001. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

