RNA polymerase subunits encoded by the plastid rpo genes are not shared with the nucleus-encoded plastid enzyme
- PMID: 9701572
- PMCID: PMC34880
- DOI: 10.1104/pp.117.4.1165
RNA polymerase subunits encoded by the plastid rpo genes are not shared with the nucleus-encoded plastid enzyme
Abstract
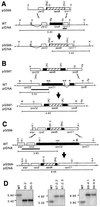
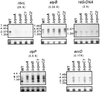
Plastid genes in photosynthetic higher plants are transcribed by at least two RNA polymerases. The plastid rpoA, rpoB, rpoC1, and rpoC2 genes encode subunits of the plastid-encoded plastid RNA polymerase (PEP), an Escherichia coli-like core enzyme. The second enzyme is referred to as the nucleus-encoded plastid RNA polymerase (NEP), since its subunits are assumed to be encoded in the nucleus. Promoters for NEP have been previously characterized in tobacco plants lacking PEP due to targeted deletion of rpoB (encoding the beta-subunit) from the plastid genome. To determine if NEP and PEP share any essential subunits, the rpoA, rpoC1, and rpoC2 genes encoding the PEP alpha-, beta'-, and beta"-subunits were removed by targeted gene deletion from the plastid genome. We report here that deletion of each of these genes yielded photosynthetically defective plants that lack PEP activity while maintaining transcription specificity from NEP promoters. Therefore, rpoA, rpoB, rpoC1, and rpoC2 encode PEP subunits that are not essential components of the NEP transcription machinery. Furthermore, our data indicate that no functional copy of rpoA, rpoB, rpoC1, or rpoC2 that could complement the deleted plastid rpo genes exists outside the plastids.
Figures


References
-
- Gruissem W, Tonkyn JC. Control mechanisms of plastid gene expression. Crit Rev Plant Sci. 1993;12:19–55.
-
- Hedtke B, Börner T, Weihe A. Mitochondrial and chloroplast phage-type RNA polymerases in Arabidopsis. Science. 1997;277:809–811. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

