Control of meristem development by CLAVATA1 receptor kinase and kinase-associated protein phosphatase interactions
- PMID: 9701578
- PMCID: PMC34886
- DOI: 10.1104/pp.117.4.1217
Control of meristem development by CLAVATA1 receptor kinase and kinase-associated protein phosphatase interactions
Abstract
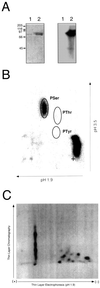
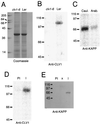
The CLAVATA1 (CLV1) gene encodes a putative receptor kinase required for the proper balance between cell proliferation and differentiation in Arabidopsis shoot and flower meristems. Impaired CLV1 signaling results in masses of undifferentiated cells at the shoot and floral meristems. Although many putative receptor kinases have been identified in plants, the mechanism of signal transduction mediated by plant receptor-like kinases is largely unknown. One potential effector of receptor kinase signaling is kinase-associated protein phosphatase (KAPP), a protein that binds to multiple plant receptor-like kinases in a phosphorylation-dependent manner. To examine a possible role for KAPP in CLV1-dependent plant development, the interaction of CLV1 and KAPP was investigated in vitro and in vivo. KAPP binds directly to autophosphorylated CLV1 in vitro and co-immunoprecipitates with CLV1 in plant extracts derived from meristematic tissue. Reduction of KAPP transcript accumulation in an intermediate clv1 mutant suppresses the mutant phenotype, and the degree of suppression is inversely correlated with KAPP mRNA levels. These data suggest that KAPP functions as a negative regulator of CLV1 signaling in plant development. This may represent a general model for the interaction of KAPP with receptor kinases.
Figures


References
-
- Bechtold N, Ellis J, Pelletier G. In plantaAgrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. CR Acad Sci Paris Life Sci. 1993;316:1194–1199.
-
- Boyle WJ, van der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

