Polygalacturonase-mediated solubilization and depolymerization of pectic polymers in tomato fruit cell walls . Regulation By ph and ionic conditions
- PMID: 9701584
- PMCID: PMC34892
- DOI: 10.1104/pp.117.4.1293
Polygalacturonase-mediated solubilization and depolymerization of pectic polymers in tomato fruit cell walls . Regulation By ph and ionic conditions
Abstract
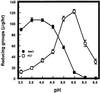
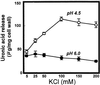
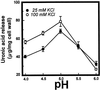
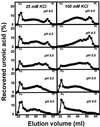
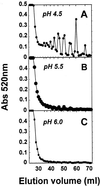
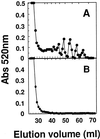
The hydrolysis of cell wall pectins by tomato (Lycopersicon esculentum) polygalacturonase (PG) in vitro is more extensive than the degradation affecting these polymers during ripening. We examined the hydrolysis of polygalacturonic acid and cell walls by PG isozyme 2 (PG2) under conditions widely adopted in the literature (pH 4.5 and containing Na+) and under conditions approximating the apoplastic environment of tomato fruit (pH 6.0 and K+ as the predominate cation). The pH optima for PG2 in the presence of K+ were 1.5 and 0.5 units higher for the hydrolysis of polygalacturonic acid and cell walls, respectively, compared with activity in the presence of Na+. Increasing K+ concentration stimulated pectin solubilization at pH 4.5 but had little influence at pH 6.0. Pectin depolymerization by PG2 was extensive at pH values from 4.0 to 5.0 and was further enhanced at high K+ levels. Oligomers were abundant products in in vitro reactions at pH 4.0 to 5.0, decreased sharply at pH 5.5, and were negligible at pH 6.0. EDTA stimulated PG-mediated pectin solubilization at pH 6.0 but did not promote oligomer production. Ca2+ suppressed PG-mediated pectin release at pH 4.5 yet had minimal influence on the proportional recovery of oligomers. Extensive pectin breakdown in processed tomato might be explained in part by cation- and low-pH-induced stimulation of PG and other wall-associated enzymes.
Figures


References
-
- Ali ZM, Brady CJ. Purification and characterization of the polygalacturonases of tomato fruits. Aust J Plant Physiol. 1982;9:155–169.
-
- Al-Shaibani AMH, Greig JK. Effects of stage of maturity, storage, and cultivar on some quality attributes of tomatoes. J Am Soc Hortic Sci. 1979;104:880–882.
-
- Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54:484–489. - PubMed
-
- Burns JK, Pressey R. Enhancement of the release of protoplasts and pectin from tomato locular gel by pectin methylesterase. J Am Soc Hortic Sci. 1988;113:624–626.
-
- Chun J-P, Huber DJ. Polygalacturonase isozyme 2 binding and catalysis in cell walls from tomato fruit: pH and β-subunit effects. Physiol Plant. 1997;101:283–290.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

