Molecular characterization of an Arabidopsis gene encoding hydroperoxide lyase, a cytochrome P-450 that is wound inducible
- PMID: 9701595
- PMCID: PMC34903
- DOI: 10.1104/pp.117.4.1393
Molecular characterization of an Arabidopsis gene encoding hydroperoxide lyase, a cytochrome P-450 that is wound inducible
Abstract
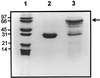
Hydroperoxide lyase (HPL) cleaves lipid hydroperoxides to produce volatile flavor molecules and also potential signal molecules. We have characterized a gene from Arabidopsis that is homologous to a recently cloned HPL from green pepper (Capsicum annuum). The deduced protein sequence indicates that this gene encodes a cytochrome P-450 with a structure similar to that of allene oxide synthase. The gene was cloned into an expression vector and expressed in Escherichia coli to demonstrate HPL activity. Significant HPL activity was evident when 13S-hydroperoxy-9(Z),11(E),15(Z)-octadecatrienoic acid was used as the substrate, whereas activity with 13S-hydroperoxy-9(Z),11(E)-octadecadienoic acid was approximately 10-fold lower. Analysis of headspace volatiles by gas chromatography-mass spectrometry, after addition of the substrate to E. coli extracts expressing the protein, confirmed enzyme-activity data, since cis-3-hexenal was produced by the enzymatic activity of the encoded protein, whereas hexanal production was limited. Molecular characterization of this gene indicates that it is expressed at high levels in floral tissue and is wound inducible but, unlike allene oxide synthase, it is not induced by treatment with methyl jasmonate.
Figures




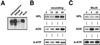
References
-
- Altschul SF, Gish W, Miller W, Myers W, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Anderson JM (1989) Membrane-derived fatty acids as precursors to second messengers. In WF Smith, DJ Morr, eds, Second Messengers in Plant Growth and Development, Vol 6. Alan R Liss, New York, pp 181–212
-
- Archbold DD, Hamilton-Kemp TR, Barth MM, Langlois BE. Identifying natural volatile compounds that control gray mold (Botrytis cinera) during postharvest storage of strawberry, blackberry, and grape. J Agric Food Chem. 1997;45:4032–4037.
-
- Ausubel FM, Brent R, Kingston RE, Moore DM, Seidman JG, Smith JA, Struhl K (1987) Current Protocols in Molecular Biology. Wiley, New York
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

