Mechanistic independence of Nef and cyclophilin A enhancement of human immunodeficiency virus type 1 infectivity
- PMID: 9705263
- PMCID: PMC3937310
- DOI: 10.1006/viro.1998.9254
Mechanistic independence of Nef and cyclophilin A enhancement of human immunodeficiency virus type 1 infectivity
Abstract
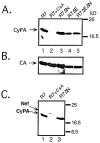
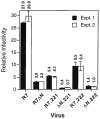
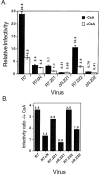
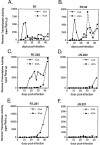
Optimal HIV-1 infectivity requires the presence of both the viral factor Nef and the cellular protein cyclophilin A (CyPA) during virion assembly. These two proteins are integral components of HIV-1 particles. Both CyPA and Nef facilitate a step in the viral life cycle occurring between penetration and reverse transcription, suggesting a common mechanism of action. Experiments were performed to test the potential interplay of Nef- and CyPA-mediated enhancement of HIV-1 infectivity. In single-cycle infection assays, nef-defective virions were partially resistant to cyclosporin A (CsA), a drug that inhibits the binding of CyPA to the HIV-1 Gag precursor and CyPA incorporation into virions. Genetic dissection of the relative contributions of Nef and the cyclophilin A-Gag interaction to HIV-1 infectivity demonstrated the independence of these two effects. Nef was not required for incorporation of CyPA into HIV-1 virions and vice-versa. Surprisingly, CyPA-deficient virions remained sensitive to inhibition by CsA, in a manner that depended strongly on the presence of a functional nef gene. These results demonstrate that Nef and CyPA act independently to render HIV-1 particles fully infectious. They further suggest that in addition to blocking the CyPA-Gag interaction, CsA can also inhibit HIV-1 replication through a novel mechanism involving suppression of Nef-directed enhancement of virus infectivity.
Copyright 1998 Academic Press.
Figures


References
-
- Aiken C, Konner J, Landau NR, Lenburg ME, Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76:853–864. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

