The gamma interferon gene knockout mouse: a highly sensitive model for evaluation of therapeutic agents against Cryptosporidium parvum
- PMID: 9705383
- PMCID: PMC105153
- DOI: 10.1128/JCM.36.9.2503-2508.1998
The gamma interferon gene knockout mouse: a highly sensitive model for evaluation of therapeutic agents against Cryptosporidium parvum
Abstract
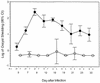
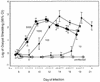
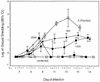
Cryptosporidiosis is a serious disease in malnourished children and in people with malignancies or AIDS. Current rodent models for evaluating drug therapy against cryptosporidiosis have many limitations, including the need for a high inoculum, the absence of symptoms resembling those seen in humans, and the need to maintain exogenous immunosuppression. We have developed a gamma interferon knockout (GKO) mouse model with which to evaluate therapies against C. parvum and have used paromomycin for evaluation of this model. The GKO model offers considerable improvements over other systems, since it requires no additional immunosuppression and adult mice can be infected with as few as 10 oocysts (compared with 10(7) for SCID mice). Infected mice develop profound gastrointestinal dysfunction due to extensive infection and severe mucosal damage involving the entire small intestine. Clinical symptoms, which include depression, anorexia, weight loss, and wasting, result in death within 2 to 4 weeks. The time of death depends on the oocyst challenge dose. Paromomycin modulated parasitological and clinical parameters in highly predictable and significant ways, including prevention of death. In addition, examination of the extensively infected gut provided an important insight into the dynamics between a specific drug treatment, its impact on the extent and the site of parasite distribution, and clinical outcome. These uniform symptoms of weight loss, wasting, and death are powerful new parameters which bring this model closer to the actual disease seen in humans and other susceptible mammalian species.
Figures


Similar articles
-
Cryptosporidium parvum-specific mucosal immune response in C57BL/6 neonatal and gamma interferon-deficient mice: role of tumor necrosis factor alpha in protection.Infect Immun. 2001 Mar;69(3):1635-42. doi: 10.1128/IAI.69.3.1635-1642.2001. Infect Immun. 2001. PMID: 11179338 Free PMC article.
-
Cytokine expression and specific lymphocyte proliferation in two strains of Cryptosporidium parvum-infected gamma-interferon knockout mice.J Parasitol. 2000 Apr;86(2):300-7. doi: 10.1645/0022-3395(2000)086[0300:CEASLP]2.0.CO;2. J Parasitol. 2000. PMID: 10780549
-
Profiles of healing and nonhealing Cryptosporidium parvum infection in C57BL/6 mice with functional B and T lymphocytes: the extent of gamma interferon modulation determines the outcome of infection.Infect Immun. 1997 Nov;65(11):4761-9. doi: 10.1128/iai.65.11.4761-4769.1997. Infect Immun. 1997. PMID: 9353062 Free PMC article.
-
Treatment of cryptosporidial diarrhea in an AIDS patient with paromomycin.Ann Pharmacother. 1993 Dec;27(12):1460-2. doi: 10.1177/106002809302701209. Ann Pharmacother. 1993. PMID: 8305777 Review.
-
Human cryptosporidiosis.Int J STD AIDS. 1996;7 Suppl 1:28-33. doi: 10.1258/0956462961917285. Int J STD AIDS. 1996. PMID: 8652725 Review.
Cited by
-
Inactivation of exogenous endoparasite stages by chemical disinfectants: current state and perspectives.Parasitol Res. 2013 Mar;112(3):917-32. doi: 10.1007/s00436-013-3324-4. Epub 2013 Feb 8. Parasitol Res. 2013. PMID: 23392903 Review.
-
New insights into human cryptosporidiosis.Clin Microbiol Rev. 1999 Oct;12(4):554-63. doi: 10.1128/CMR.12.4.554. Clin Microbiol Rev. 1999. PMID: 10515902 Free PMC article. Review.
-
Human intestinal and biliary cryptosporidiosis.World J Gastroenterol. 1999 Oct;5(5):424-429. doi: 10.3748/wjg.v5.i5.424. World J Gastroenterol. 1999. PMID: 11819481 Free PMC article. No abstract available.
-
A suite of phenotypic assays to ensure pipeline diversity when prioritizing drug-like Cryptosporidium growth inhibitors.Nat Commun. 2019 Apr 23;10(1):1862. doi: 10.1038/s41467-019-09880-w. Nat Commun. 2019. PMID: 31015448 Free PMC article.
-
A cysteine protease inhibitor rescues mice from a lethal Cryptosporidium parvum infection.Antimicrob Agents Chemother. 2013 Dec;57(12):6063-73. doi: 10.1128/AAC.00734-13. Epub 2013 Sep 23. Antimicrob Agents Chemother. 2013. PMID: 24060869 Free PMC article.
References
-
- Blagburn B L, Soave R. Prophylaxis and chemotherapy: human and animal. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis—1997. Boca Raton, Fla: CRC Press; 1997. pp. 111–128.
-
- Current W L. Techniques and laboratory maintenance of Cryptosporidium. In: Dubey J P, Speer C A, Fayer R, editors. Cryptosporidiosis of man and animals—1990. Boston, Mass: CRC Press; 1990. pp. 31–49.
-
- Davis L J, Soave R, Dudley R E, Fessel J W, Faulkner S, Mamakos J P. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Nitazoxanide (NTZ) for AIDS-related cryptosporidial diarrhea (CD): an open-label safety, efficacy and pharmacokinetic study, abstr. LM50; p. 289.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

