Interleukin 3 prevents delayed neuronal death in the hippocampal CA1 field
- PMID: 9705946
- PMCID: PMC2213360
- DOI: 10.1084/jem.188.4.635
Interleukin 3 prevents delayed neuronal death in the hippocampal CA1 field
Abstract
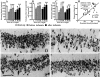
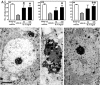
In the central nervous system, interleukin (IL)-3 has been shown to exert a trophic action only on septal cholinergic neurons in vitro and in vivo, but a widespread distribution of IL-3 receptor (IL-3R) in the brain does not conform to such a selective central action of the ligand. Moreover, the mechanism(s) underlying the neurotrophic action of IL-3 has not been elucidated, although an erythroleukemic cell line is known to enter apoptosis after IL-3 starvation possibly due to a rapid decrease in Bcl-2 expression. This in vivo study focused on whether IL-3 rescued noncholinergic hippocampal neurons from lethal ischemic damage by modulating the expression of Bcl-xL, a Bcl-2 family protein produced in the mature brain. 7-d IL-3 infusion into the lateral ventricle of gerbils with transient forebrain ischemia prevented significantly hippocampal CA1 neuron death and ischemia-induced learning disability. TUNEL (terminal deoxynucleotidyltransferase-mediated 2'-deoxyuridine 5'-triphosphate-biotin nick end labeling) staining revealed that IL-3 infusion caused a significant reduction in the number of CA1 neurons exhibiting DNA fragmentation 7 d after ischemia. The neuroprotective action of IL-3 appeared to be mediated by a postischemic transient upregulation of the IL-3R alpha subunit in the hippocampal CA1 field where IL-3Ralpha was barely detectable under normal conditions. In situ hybridization histochemistry and immunoblot analysis demonstrated that Bcl-xL mRNA expression, even though upregulated transiently in CA1 pyramidal neurons after ischemia, did not lead to the production of Bcl-xL protein in ischemic gerbils infused with vehicle. However, IL-3 infusion prevented the decrease in Bcl-xL protein expression in the CA1 field of ischemic gerbils. Subsequent in vitro experiments showed that IL-3 induced the expression of Bcl-xL mRNA and protein in cultured neurons with IL-3Ralpha and attenuated neuronal damage caused by a free radical-producing agent FeSO4. These findings suggest that IL-3 prevents delayed neuronal death in the hippocampal CA1 field through a receptor-mediated expression of Bcl-xL protein, which is known to facilitate neuron survival. Since IL-3Ralpha in the hippocampal CA1 region, even though upregulated in response to ischemic insult, is much less intensely expressed than that in the CA3 region tolerant to ischemia, the paucity of IL-3R interacting with the ligand may account for the vulnerability of CA1 neurons to ischemia.
Figures





Similar articles
-
Erythropoietin protects neurons against chemical hypoxia and cerebral ischemic injury by up-regulating Bcl-xL expression.J Neurosci Res. 2002 Mar 15;67(6):795-803. doi: 10.1002/jnr.10166. J Neurosci Res. 2002. PMID: 11891794
-
Epidermal growth factor protects neuronal cells in vivo and in vitro against transient forebrain ischemia- and free radical-induced injuries.J Cereb Blood Flow Metab. 1998 Apr;18(4):349-60. doi: 10.1097/00004647-199804000-00002. J Cereb Blood Flow Metab. 1998. PMID: 9538899
-
Protective effect of a prosaposin-derived, 18-mer peptide on slowly progressive neuronal degeneration after brief ischemia.J Cereb Blood Flow Metab. 2001 Nov;21(11):1295-302. doi: 10.1097/00004647-200111000-00005. J Cereb Blood Flow Metab. 2001. PMID: 11702044
-
Selective vulnerability of hippocampal CA1 and CA3 pyramidal cells: What are possible pathomechanisms and should more attention be paid to the CA3 region in future studies?J Neurosci Res. 2024 Jan;102(1):e25276. doi: 10.1002/jnr.25276. J Neurosci Res. 2024. PMID: 38284845 Review.
-
(-)Deprenyl reduces delayed neuronal death of hippocampal pyramidal cells.Neurosci Biobehav Rev. 1997 Mar;21(2):181-6. doi: 10.1016/s0149-7634(96)00008-5. Neurosci Biobehav Rev. 1997. PMID: 9062941 Review.
Cited by
-
Transplantation of human placental chorionic plate-derived mesenchymal stem cells for repair of neurological damage in neonatal hypoxic-ischemic encephalopathy.Neural Regen Res. 2024 Sep 1;19(9):2027-2035. doi: 10.4103/1673-5374.390952. Epub 2023 Dec 15. Neural Regen Res. 2024. PMID: 38227532 Free PMC article.
-
Enhanced prospects for drug delivery and brain targeting by the choroid plexus-CSF route.Pharm Res. 2005 Jul;22(7):1011-37. doi: 10.1007/s11095-005-6039-0. Epub 2005 Jul 22. Pharm Res. 2005. PMID: 16028003 Review.
-
Abnormal Changes of IL3/IL3R and Its Downstream Signaling Pathways in the Prion-Infected Cell Line and in the Brains of Scrapie-Infected Rodents.Mol Neurobiol. 2024 Dec;61(12):9756-9775. doi: 10.1007/s12035-023-03511-8. Epub 2023 Aug 7. Mol Neurobiol. 2024. PMID: 37548852
-
A cytokine mixture of GM-CSF and IL-3 that induces a neuroprotective phenotype of microglia leading to amelioration of (6-OHDA)-induced Parkinsonism of rats.Brain Behav. 2011 Sep;1(1):26-43. doi: 10.1002/brb3.11. Brain Behav. 2011. PMID: 22398979 Free PMC article.
-
Adaptive evolution of interleukin-3 (IL3), a gene associated with brain volume variation in general human populations.Hum Genet. 2016 Apr;135(4):377-392. doi: 10.1007/s00439-016-1644-z. Epub 2016 Feb 13. Hum Genet. 2016. PMID: 26875095
References
-
- Schrader JW. The pan specific hemopoietin of activated T-lymphocytes (interleukin-3) Annu Rev Immunol. 1986;4:205–230. - PubMed
-
- Frendl G, Beller DI. Regulation of macrophage activation by IL-3. J Immunol. 1990;144:3392–3399. - PubMed
-
- Frendl G. Interleukin-3: from colony-stimulating factor to pluripotent immunoregulatory cytokine. J Immunol. 1992;14:421–430. - PubMed
-
- Ihle JN, Weinstein Y. Immunological regulation of hematopoietic/lymphoid stem cell differentiation by interleukin 3. Adv Immunol. 1986;39:1–50. - PubMed
-
- Clarke SC, Kamen R. The human hematopoi-etic colony-stimulating factors. Science. 1987;236:1229–1237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

