Angiostatin-mediated suppression of cancer metastases by primary neoplasms engineered to produce granulocyte/macrophage colony-stimulating factor
- PMID: 9705957
- PMCID: PMC2213351
- DOI: 10.1084/jem.188.4.755
Angiostatin-mediated suppression of cancer metastases by primary neoplasms engineered to produce granulocyte/macrophage colony-stimulating factor
Abstract
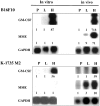
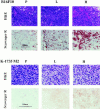
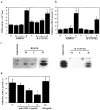
We determined whether tumor cells consistently generating granulocyte/macrophage colony- stimulating factor (GM-CSF) can recruit and activate macrophages to generate angiostatin and, hence, inhibit the growth of distant metastasis. Two murine melanoma lines, B16-F10 (syngeneic to C57BL/6 mice) and K-1735 (syngeneic to C3H/HeN mice), were engineered to produce GM-CSF. High GM-CSF (>1 ng/10(6) cells)- and low GM-CSF (<10 pg/10(6) cells)-producing clones were identified. Parental, low, and high GM-CSF-producing cells were injected subcutaneously into syngeneic and into nude mice. Parental and low-producing cells produced rapidly growing tumors, whereas the high-producing cells produced slow-growing tumors. Macrophage density inversely correlated with tumorigenicity and directly correlated with steady state levels of macrophage metalloelastase (MME) mRNA. B16 and K-1735 subcutaneous (s.c.) tumors producing high levels of GM-CSF significantly suppressed lung metastasis of 3LL, UV-2237 fibrosarcoma, K-1735 M2, and B16-F10 cells, but parental or low-producing tumors did not. The level of angiostatin in the serum directly correlated with the production of GM-CSF by the s.c. tumors. Macrophages incubated with medium conditioned by GM-CSF- producing B16 or K-1735 cells had higher MME activity and generated fourfold more angiostatin than control counterparts. These data provide direct evidence that GM-CSF released from a primary tumor can upregulate angiostatin production and suppress growth of metastases.
Figures

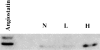
Similar articles
-
Macrophage-derived metalloelastase is responsible for the generation of angiostatin in Lewis lung carcinoma.Cell. 1997 Mar 21;88(6):801-10. doi: 10.1016/s0092-8674(00)81926-1. Cell. 1997. PMID: 9118223
-
GM-CSF-transduced B16 melanoma cells are highly susceptible to lysis by normal murine macrophages and poorly tumorigenic in immune-compromised mice.J Leukoc Biol. 1999 Jan;65(1):102-8. doi: 10.1002/jlb.65.1.102. J Leukoc Biol. 1999. PMID: 9886252
-
Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation.J Exp Med. 1999 Aug 2;190(3):355-66. doi: 10.1084/jem.190.3.355. J Exp Med. 1999. PMID: 10430624 Free PMC article.
-
Pulsing of dendritic cells with cell lysates from either B16 melanoma or MCA-106 fibrosarcoma yields equally effective vaccines against B16 tumors in mice.J Surg Oncol. 1998 Jun;68(2):79-91. doi: 10.1002/(sici)1096-9098(199806)68:2<79::aid-jso3>3.0.co;2-h. J Surg Oncol. 1998. PMID: 9624036 Review.
-
[A correlation between the abilities of tumor cells to produce GM-CSF and form metastasis].Gan To Kagaku Ryoho. 1989 Oct;16(10):3367-73. Gan To Kagaku Ryoho. 1989. PMID: 2679394 Review. Japanese.
Cited by
-
WTC-01, a novel synthetic oxime-flavone compound, destabilizes microtubules in human nasopharyngeal carcinoma cells in vitro and in vivo.Br J Pharmacol. 2015 Oct;172(19):4671-83. doi: 10.1111/bph.13227. Epub 2015 Aug 14. Br J Pharmacol. 2015. PMID: 26102991 Free PMC article.
-
The role of the tumor microenvironment in regulating angiogenesis.Cold Spring Harb Perspect Med. 2012 Dec 1;2(12):a006676. doi: 10.1101/cshperspect.a006676. Cold Spring Harb Perspect Med. 2012. PMID: 23209177 Free PMC article.
-
Angiogenesis, metastasis, and endogenous inhibition.J Neurooncol. 2000 Oct-Nov;50(1-2):173-80. doi: 10.1023/a:1006453428013. J Neurooncol. 2000. PMID: 11245277 Review.
-
Therapeutic cancer vaccines: past, present, and future.Adv Cancer Res. 2013;119:421-75. doi: 10.1016/B978-0-12-407190-2.00007-1. Adv Cancer Res. 2013. PMID: 23870514 Free PMC article. Review.
-
Chitosan hydrogel containing GMCSF and a cancer drug exerts synergistic anti-tumor effects via the induction of CD8+ T cell-mediated anti-tumor immunity.Clin Exp Metastasis. 2009;26(3):179-87. doi: 10.1007/s10585-008-9228-5. Epub 2008 Dec 14. Clin Exp Metastasis. 2009. PMID: 19082918
References
-
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. - PubMed
-
- Folkman J. Clinical application of research on angiogenesis. N Engl J Med. 1995;333:1753–1763. - PubMed
-
- Auerbach W, Auerbach R. Angiogenesis inhibition: a review. Pharmacol Ther. 1994;63:265–311. - PubMed
-
- Folkman J, Klagsburn M. Angiogenic factors. Science. 1987;235:444–447. - PubMed
-
- Cockerill GW, Gamble JR, Vadas MA. Angiogenesis: models and modulators. Int Rev Cytol. 1995;159:113–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

