Antigen receptor engagement turns off the V(D)J recombination machinery in human tonsil B cells
- PMID: 9705958
- PMCID: PMC2213359
- DOI: 10.1084/jem.188.4.765
Antigen receptor engagement turns off the V(D)J recombination machinery in human tonsil B cells
Abstract
The germinal center (GC) is an anatomic compartment found in peripheral lymphoid organs, wherein B cells undergo clonal expansion, somatic mutation, switch recombination, and reactivate immunoglobulin gene V(D)J recombination. As a result of somatic mutation, some GC B cells develop higher affinity antibodies, whereas others suffer mutations that decrease affinity, and still others may become self-reactive. It has been proposed that secondary V(D)J rearrangements in GCs might rescue B cells whose receptors are damaged by somatic mutations. Here we present evidence that mature human tonsil B cells coexpress conventional light chains and recombination associated genes, and that they extinguish recombination activating gene and terminal deoxynucleotidyl transferase expression when their receptors are cross-linked. Thus, the response of the recombinase to receptor engagement in peripheral B cells is the opposite of the response in developing B cells to the same stimulus. These observations suggest that receptor revision is a mechanism for receptor diversification that is turned off when antigen receptors are cross-linked by the cognate antigen.
Figures



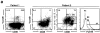
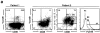




Similar articles
-
RAG1 and RAG2 expression by B cell subsets from human tonsil and peripheral blood.J Immunol. 2001 Jan 1;166(1):377-86. doi: 10.4049/jimmunol.166.1.377. J Immunol. 2001. PMID: 11123315
-
Secondary V(D)J rearrangements and B cell receptor-mediated down-regulation of recombination activating gene-2 expression in a murine B cell line.J Immunol. 2000 Jul 15;165(2):703-9. doi: 10.4049/jimmunol.165.2.703. J Immunol. 2000. PMID: 10878342
-
V(D)J recombinase activity in a subset of germinal center B lymphocytes.Science. 1997 Oct 10;278(5336):301-5. doi: 10.1126/science.278.5336.301. Science. 1997. PMID: 9323211
-
Factors and forces controlling V(D)J recombination.Adv Immunol. 2001;78:169-232. doi: 10.1016/s0065-2776(01)78004-2. Adv Immunol. 2001. PMID: 11432204 Review. No abstract available.
-
RAG1 and RAG2 in V(D)J recombination and transposition.Immunol Res. 2001;23(1):23-39. doi: 10.1385/IR:23:1:23. Immunol Res. 2001. PMID: 11417858 Review.
Cited by
-
Receptor revision of immunoglobulin heavy chain variable region genes in normal human B lymphocytes.J Exp Med. 2000 Jun 5;191(11):1881-94. doi: 10.1084/jem.191.11.1881. J Exp Med. 2000. PMID: 10839804 Free PMC article.
-
Self-antigen recognition by follicular lymphoma B-cell receptors.Blood. 2012 Nov 15;120(20):4182-90. doi: 10.1182/blood-2012-05-427534. Epub 2012 Sep 28. Blood. 2012. PMID: 23024238 Free PMC article.
-
Recombination activating genes (RAG) induce secondary Ig gene rearrangement in and subsequent apoptosis of human peripheral blood circulating B lymphocytes.Clin Exp Immunol. 2004 Apr;136(1):76-84. doi: 10.1111/j.1365-2249.2004.02423.x. Clin Exp Immunol. 2004. PMID: 15030517 Free PMC article.
-
Neutralizing antiviral antibody responses.Adv Immunol. 2001;79:1-53. doi: 10.1016/s0065-2776(01)79001-3. Adv Immunol. 2001. PMID: 11680006 Free PMC article. Review.
-
Patterns of receptor revision in the immunoglobulin heavy chains of a teleost fish.J Immunol. 2009 May 1;182(9):5605-22. doi: 10.4049/jimmunol.0801013. J Immunol. 2009. PMID: 19380808 Free PMC article.
References
-
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:575–581. - PubMed
-
- Willerford DM, Swat W, Alt FW. Developmental regulation of V(D)J recombination and lymphocyte differentiation. Curr Opin Genet Dev. 1996;6:603–609. - PubMed
-
- Schatz DG, Oettinger MA, Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989;59:1035–1048. - PubMed
-
- Oettinger MA, Schatz DG, Gorka C, Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990;248:1517–1523. - PubMed
-
- Shinkai Y, Rathbun G, Lam K-P, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, Alt FW. RAG-2 deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

