The sulphonylurea receptor SUR1 regulates ATP-sensitive mouse Kir6.2 K+ channels linked to the green fluorescent protein in human embryonic kidney cells (HEK 293)
- PMID: 9705987
- PMCID: PMC2231056
- DOI: 10.1111/j.1469-7793.1998.333bk.x
The sulphonylurea receptor SUR1 regulates ATP-sensitive mouse Kir6.2 K+ channels linked to the green fluorescent protein in human embryonic kidney cells (HEK 293)
Abstract
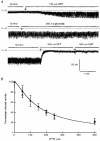
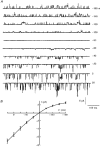
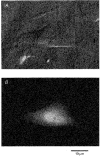
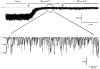
1. Using a chimeric protein comprising the green fluorescent protein (GFP) linked to the C-terminus of the K+ channel protein mouse Kir6.2 (Kir6.2-C-GFP), the interactions between the sulphonylurea receptor SUR1 and Kir6.2 were investigated in transfected human embryonic kidney cells (HEK 293) by combined imaging and patch clamp techniques. 2. HEK 293 cells transfected with mouse Kir6.2-C-GFP and wild-type Kir6.2 exhibited functional K+ channels independently of SUR1. These channels were inhibited by ATP (IC50 = 150 microM), but were not responsive to stimulation by ADP or inhibition by sulphonylureas. Typically 15 +/- 7 active channels were found in an excised patch. 3. The distribution of Kir6.2-C-GFP protein was investigated by imaging of GFP fluorescence. There was a lamellar pattern of fluorescence labelling inside the cytoplasm (presumably associated with the endoplasmic reticulum and the Golgi apparatus) and intense punctate labelling near the cell membrane, but little fluorescence was associated with the plasma membrane. 4. In contrast, cells co-transfected with Kir6.2-C-GFP and SUR1 exhibited intense uniform plasma membrane labelling, and the lamellar and punctate labelling seen without SUR1 was no longer prominent. 5. In cells co-transfected with Kir6.2-C-GFP and SUR1, strong membrane labelling was associated with very high channel activity, with 484 +/- 311 active channels per excised patch. These K+ channels were sensitive to inhibition by ATP (IC50 = 17 microM), stimulated by ADP and inhibited by sulphonylureas. 6. We conclude that co-expression of SUR1 and Kir6.2 generates channels with the properties of native KATP channels. In addition, SUR1 promotes uniform insertion of Kir6.2-C-GFP into the plasma membrane and a 35-fold increase in channel activity, suggesting that SUR1 facilitates protein trafficking of Kir6.2 into the plasma membrane.
Figures



References
-
- Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, IV, Boyd AE, III, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson DA. Cloning of the β cell high-affinity sulfonylurea receptor: A regulator of insulin secretion. Science. 1995;268:423–426. - PubMed
-
- Ashcroft FM. Adenosine 5′-triphosphate-sensitive potassium channels. Annual Review of Neuroscience. 1988;11:97–118. 10.1146/annurev.ne.11.030188.000525. - DOI - PubMed
-
- Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. - PubMed
-
- Cockett MI, Ochalski R, Benwell K, Franco R, Wardell-Swanson J. Simultaneous expression of multi-subunit proteins in mammalian cells using a convenient set of mammalian cell expression vectors. Biotechniques. 1977;23:402–405. - PubMed
-
- Daut J, Maier-Rudolph W, Von Beckerath N, Mehrke G, Gunther K. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science. 1990;247:1341–1344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

