The effect of overexpression of auxiliary Ca2+ channel subunits on native Ca2+ channel currents in undifferentiated mammalian NG108-15 cells
- PMID: 9705988
- PMCID: PMC2231049
- DOI: 10.1111/j.1469-7793.1998.347bk.x
The effect of overexpression of auxiliary Ca2+ channel subunits on native Ca2+ channel currents in undifferentiated mammalian NG108-15 cells
Abstract
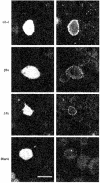
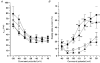
1. High voltage activated (HVA) Ca2+ channels are composed of a pore-forming alpha 1 subunit and the accessory beta and alpha2-delta subunits. However, the subunit composition of low voltage activated (LVA), or T-type, Ca2+ channels has yet to be elucidated. We have examined whether native calcium channels in NG108-15 mouse neuroblastoma x rat glioma hybrid cells, which express predominantly LVA currents when undifferentiated, are modulated by overexpression of accessory calcium channel subunits. 2. Endogenous alpha 1A, B, C, C, and E, and low levels of beta and alpha 2-delta subunit protein were demonstrated in undifferentiated NG108-15 cells. 3. The alpha 2-delta, beta 2a or beta 1b accessory subunits were overexpressed by transfection of the cDNAs into these cells, and the effect examined on the endogenous Ca2+ channel currents. Heterologous expression, particularly of alpha 2-delta but also of beta 2a subunits clearly affected the profile of these currents. Both subunits induced a sustained component in the currents evoked by depolarizing voltages above -30 mV, and alpha 2-delta additionally caused a depolarization in the voltage dependence of current activation, suggesting that it also affected the native T-type currents. In contrast, beta 1b overexpression had no effect on the endogenous Ca2+ currents, despite immunocytochemical evidence for its expression in the transfected cells. 4 These results suggest that in NG108-15 cells, overexpression of the Ca2+ channel accessory subunits alpha 2-delta and beta 2a induce a sustained component of HVA current, and alpha 2-delta also influences the voltage dependence of activation of the LVA current. It is possible that native T-type alpha 1 subunits are not associated with beta subunits.
Figures



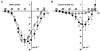
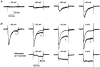



Similar articles
-
Subunit regulation of the neuronal alpha 1A Ca2+ channel expressed in Xenopus oocytes.J Physiol. 1995 Jun 15;485 ( Pt 3)(Pt 3):619-34. doi: 10.1113/jphysiol.1995.sp020757. J Physiol. 1995. PMID: 7562605 Free PMC article.
-
Calcineurin involvement in the regulation of high-threshold Ca2+ channels in NG108-15 (rodent neuroblastoma x glioma hybrid) cells.J Physiol. 1998 Jul 15;510 ( Pt 2)(Pt 2):371-85. doi: 10.1111/j.1469-7793.1998.371bk.x. J Physiol. 1998. PMID: 9705990 Free PMC article.
-
Ca2+ influx through voltage-gated Ca2+ channels regulates 5-HT3 receptor channel desensitization in rat glioma x mouse neuroblastoma hybrid NG108-15 cells.J Physiol. 1998 Jul 15;510 ( Pt 2)(Pt 2):361-70. doi: 10.1111/j.1469-7793.1998.361bk.x. J Physiol. 1998. PMID: 9705989 Free PMC article.
-
Voltage-dependent calcium channels.Gen Physiol Biophys. 2005 Jun;24 Suppl 1:1-78. Gen Physiol Biophys. 2005. PMID: 16096350 Review.
-
Emerging evidence for specific neuronal functions of auxiliary calcium channel α₂δ subunits.Gen Physiol Biophys. 2015 Apr;34(2):105-118. doi: 10.4149/gpb_2014037. Epub 2014 Dec 11. Gen Physiol Biophys. 2015. PMID: 25504062 Free PMC article. Review.
Cited by
-
The α2δ Subunit and Absence Epilepsy: Beyond Calcium Channels?Curr Neuropharmacol. 2017;15(6):918-925. doi: 10.2174/1570159X15666170309105451. Curr Neuropharmacol. 2017. PMID: 28290248 Free PMC article. Review.
-
Low-voltage-activated ("T-Type") calcium channels in review.J Bioenerg Biomembr. 2003 Dec;35(6):533-75. doi: 10.1023/b:jobb.0000008024.77488.48. J Bioenerg Biomembr. 2003. PMID: 15000520 Review.
-
Calcium signaling and T-type calcium channels in cancer cell cycling.World J Gastroenterol. 2008 Aug 28;14(32):4984-91. doi: 10.3748/wjg.14.4984. World J Gastroenterol. 2008. PMID: 18763278 Free PMC article. Review.
-
Absence of modulation of the expressed calcium channel alpha1G subunit by alpha2delta subunits.J Physiol. 1999 May 1;516 ( Pt 3)(Pt 3):639-45. doi: 10.1111/j.1469-7793.1999.0639u.x. J Physiol. 1999. PMID: 10200414 Free PMC article.
-
Tetraspanin-13 modulates voltage-gated CaV2.2 Ca2+ channels.Sci Rep. 2013;3:1777. doi: 10.1038/srep01777. Sci Rep. 2013. PMID: 23648579 Free PMC article.
References
-
- Bossu J-L, Feltz A. Patch-clamp study of the tetrodotoxin-resistant sodium current in group C sensory neurones. Neuroscience Letters. 1984;51:241–246. - PubMed
-
- Brice NL, Berrow NS, Campbell V, Page KM, Brickley K, Tedder I, Dolphin AC. Importance of the different β subunits in the membrane expression of the α1A and α2 calcium channel subunits: studies using a depolarisation-sensitive α1A antibody. European Journal of Neuroscience. 1997;9:749–759. - PubMed
-
- Brickley K, Campbell V, Berrow N, Leach R, Norman RI, Wray D, Dolphin AC, Baldwin S. Use of site-directed antibodies to probe the topography of the α2 subunit of voltage-gated Ca2+ channels. FEBS Letters. 1995;364:129–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

