Gap junctional communication and pharmacological heterogeneity in astrocytes cultured from the rat striatum
- PMID: 9705994
- PMCID: PMC2231053
- DOI: 10.1111/j.1469-7793.1998.429bk.x
Gap junctional communication and pharmacological heterogeneity in astrocytes cultured from the rat striatum
Abstract
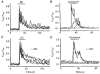
Indo-1 and fluo-3 imaging techniques were used to investigate the role of gap junctions in the changes in cytosolic calcium concentrations ([Ca2+]i) induced by several receptor agonists. Subpopulations of confluent cultured astrocytes from the rat striatum were superfused with submaximal concentrations of endothelin-1 (Et1) and the alpha 1-adrenergic and muscarinic receptor agonists, methoxamine and carbachol, respectively. 2. Combined binding and autoradiographic studies indicated that all striatal astrocytes possess binding sites for Et1. In contrast, alpha 1-adrenergic and muscarinic binding sites were found to be heterogeneously distributed. In agreement with these findings, Et1 induced fast calcium responses in all cells while only subsets of striatal astrocytes responded to the application of methoxamine or carbachol. 3. Halothane, heptanol and octanol, which are commonly used as gap junction inhibitors, drastically reduced the amplitude of Et1-induced calcium responses. In contrast, 18-alpha-glycyrrhetinic acid (alpha GA) used at a concentration known to block gap junction permeability in astrocytes had no significant effect on the amplitude of these calcium responses. 4. As demonstrated by quantitative and topological analysis, Et1 application similarly increased [Ca2+]i levels in all astrocytes in both the absence and presence of alpha GA. 5. In control conditions, subpopulations of cells responding to methoxamine or carbachol exhibited two main types of calcium responses which differed in their shape and kinetic characteristics. In the presence of alpha GA the number of cells responding to these receptor agonists was significantly reduced. Indeed, responses characterized by their long latency, slow rise time and weak amplitude disappeared in the presence of alpha GA while responses with short latency and fast rise time were preserved. 6. These results indicate that permeable gap junction channels tend to attenuate the pharmacological and functional heterogeneity of populations of astrocytes, while their inhibition restricts calcium responses in astrocytes expressing high densities of transmitter receptors coupled to phospholipase C.
Figures





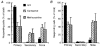
References
-
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Lodi Rizzini B, Pozzan T, Voltera A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. 10.1038/34651. - DOI - PubMed
-
- Charles AC. Glial-neuron intercellular signalling. Developmental Neuroscience. 1994;16:196–206. - PubMed
-
- Charles AC, Merrill JE, Dirksen ER, Sanderson MJ. Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron. 1991;6:983–992. - PubMed
-
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. - PubMed
-
- Dani JW, Chernjavsky A, Smith SJ. Neuronal activity triggers calcium waves in hippocampal astrocytes networks. Neuron. 1992;8:429–440. 10.1016/0896-6273(92)90271-E. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

