Role of an inward rectifier K+ current and of hyperpolarization in human myoblast fusion
- PMID: 9705997
- PMCID: PMC2231059
- DOI: 10.1111/j.1469-7793.1998.467bk.x
Role of an inward rectifier K+ current and of hyperpolarization in human myoblast fusion
Abstract
1. The role of K+ channels and membrane potential in myoblast fusion was evaluated by examining resting membrane potential and timing of expression of K+ currents at three stages of differentiation of human myogenic cells: undifferentiated myoblasts, fusion-competent myoblasts (FCMBs), and freshly formed myotubes. 2. Two K+ currents contribute to a hyperpolarization of myoblasts prior to fusion: IK(NI), a non-inactivating delayed rectifier, and IK(IR), an inward rectifier. 3. IK(NI) density is low in undifferentiated myoblasts, increases in FCMBs and declines in myotubes. On the other hand, IK(IR) is expressed in 28% of the FCMBs and in all myotubes. 4. IK(IR) is reversibly blocked by Ba2+ or Cs+. 5. Cells expressing IK(IR) have resting membrane potentials of -65 mV. A block by Ba2+ or Cs+ induces a depolarization to a voltage determined by IK(NI) (-32 mV). 6. Cs+ and Ba2+ ions reduce myoblast fusion. 7. It is hypothesized that the IK(IR)-mediated hyperpolarization allows FCMBs to recruit Na+, K+ and T-type Ca2+ channels which are present in these cells and would otherwise be inactivated. FCMBs, rendered thereby capable of firing action potentials, could amplify depolarizing signals and may accelerate fusion.
Figures
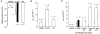
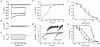


Similar articles
-
An ether -à-go-go K+ current, Ih-eag, contributes to the hyperpolarization of human fusion-competent myoblasts.J Physiol. 1998 Oct 15;512 ( Pt 2)(Pt 2):317-23. doi: 10.1111/j.1469-7793.1998.317be.x. J Physiol. 1998. PMID: 9763622 Free PMC article.
-
Contribution of a non-inactivating potassium current to the resting membrane potential of fusion-competent human myoblasts.J Physiol. 1996 May 15;493 ( Pt 1)(Pt 1):129-41. doi: 10.1113/jphysiol.1996.sp021369. J Physiol. 1996. PMID: 8735699 Free PMC article.
-
Inhibition of delayed rectifier K(+)-current by levcromakalim in single intestinal smooth muscle cells: effects of cations and dependence on K(+)-flux.Br J Pharmacol. 1995 Jan;114(2):391-9. doi: 10.1111/j.1476-5381.1995.tb13239.x. Br J Pharmacol. 1995. PMID: 7881739 Free PMC article.
-
Divalent ion block of inward rectifier current in human capillary endothelial cells and effects on resting membrane potential.J Physiol. 1998 Oct 1;512 ( Pt 1)(Pt 1):119-28. doi: 10.1111/j.1469-7793.1998.119bf.x. J Physiol. 1998. PMID: 9729622 Free PMC article.
-
Inward rectifier K+ currents in smooth muscle cells from rat coronary arteries: block by Mg2+, Ca2+, and Ba2+.Am J Physiol. 1996 Aug;271(2 Pt 2):H696-705. doi: 10.1152/ajpheart.1996.271.2.H696. Am J Physiol. 1996. PMID: 8770113
Cited by
-
Ion Channels and Transporters in Muscle Cell Differentiation.Int J Mol Sci. 2021 Dec 19;22(24):13615. doi: 10.3390/ijms222413615. Int J Mol Sci. 2021. PMID: 34948411 Free PMC article. Review.
-
Bioelectrical domain walls in homogeneous tissues.Nat Phys. 2020 Mar;16(3):357-364. doi: 10.1038/s41567-019-0765-4. Epub 2020 Jan 20. Nat Phys. 2020. PMID: 33790984 Free PMC article.
-
Sustained Depolarization of the Resting Membrane Potential Regulates Muscle Progenitor Cell Growth and Maintains Stem Cell Properties In Vitro.Stem Cell Rev Rep. 2016 Dec;12(6):634-644. doi: 10.1007/s12015-016-9687-z. Stem Cell Rev Rep. 2016. PMID: 27696329
-
Human myoblast fusion requires expression of functional inward rectifier Kir2.1 channels.J Cell Biol. 2001 May 14;153(4):677-86. doi: 10.1083/jcb.153.4.677. J Cell Biol. 2001. PMID: 11352930 Free PMC article.
-
Potassium currents in human myogenic cells from healthy and congenital myotonic dystrophy foetuses.Cell Mol Biol Lett. 2009;14(2):336-46. doi: 10.2478/s11658-009-0006-4. Epub 2009 Feb 4. Cell Mol Biol Lett. 2009. PMID: 19194665 Free PMC article.
References
-
- Baroffio A, Aubry JP, Kaelin A, Krause RM, Hamann M, Bader CR. Purification of human muscle satellite cells by flow cytometry. Muscle and Nerve. 1993;16:498–505. - PubMed
-
- Campion DR. The muscle satellite cell: a review. International Review of Cytology. 1984;87:225–251. - PubMed
-
- Cohen NA, Brenman JE, Snyder SH, Bredt DS. Binding of the inward rectifier K+ channel Kir 2.3 to PSD-95 is regulated by protein kinase A phosphorylation. Neuron. 1996;17:759–767. - PubMed
-
- Constantin B, Cognard C, Raymond G. Myoblast fusion requires cytosolic calcium elevation but not activation of voltage-dependent calcium channels. Cell Calcium. 1996;19:365–374. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

